CCR5 autogenous polypeptide vaccine and preparation method thereof
A peptide vaccine, autologous technology, applied in the field of medicine and biology, can solve the problems of B cell immune inability to break
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
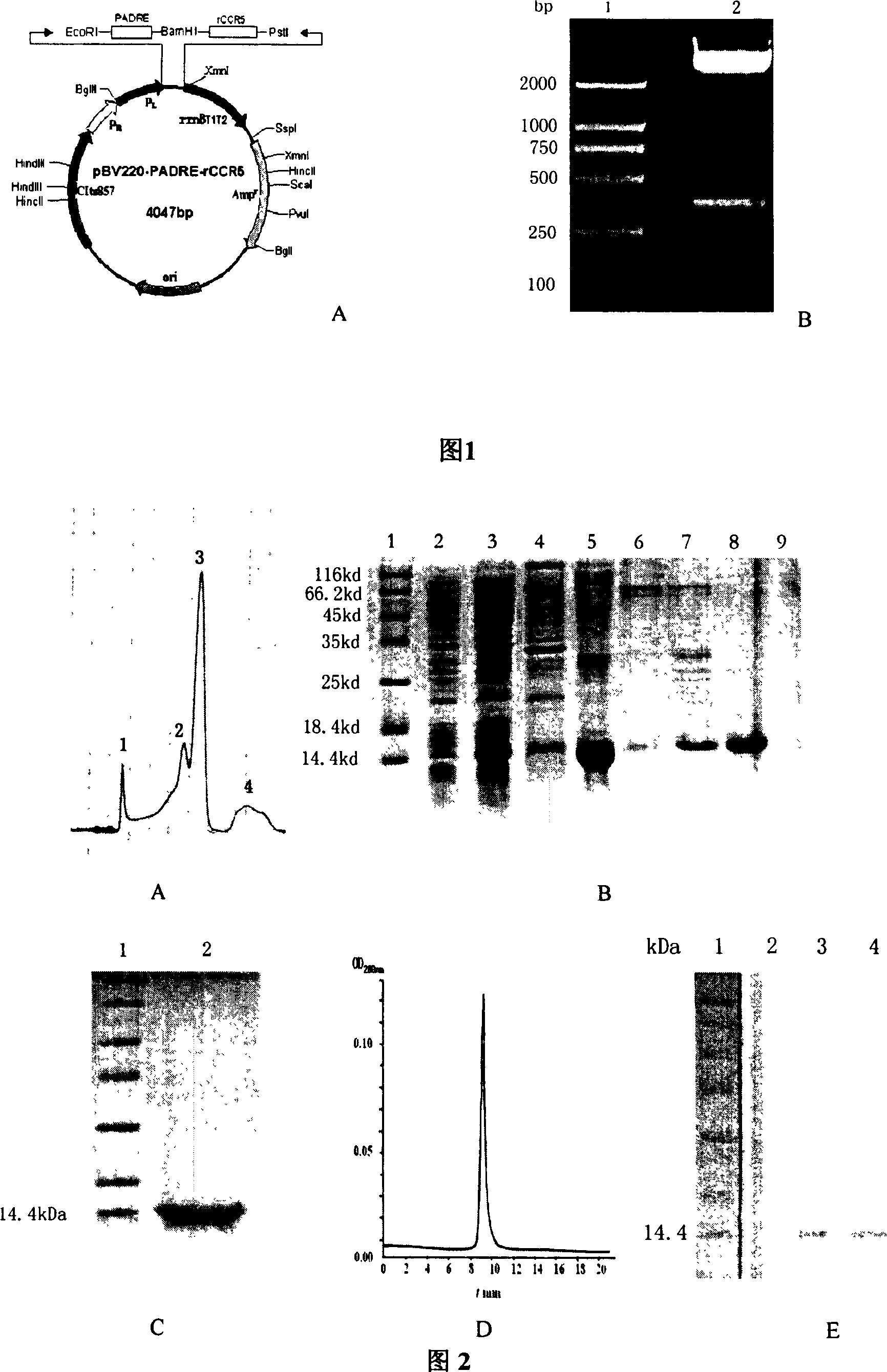
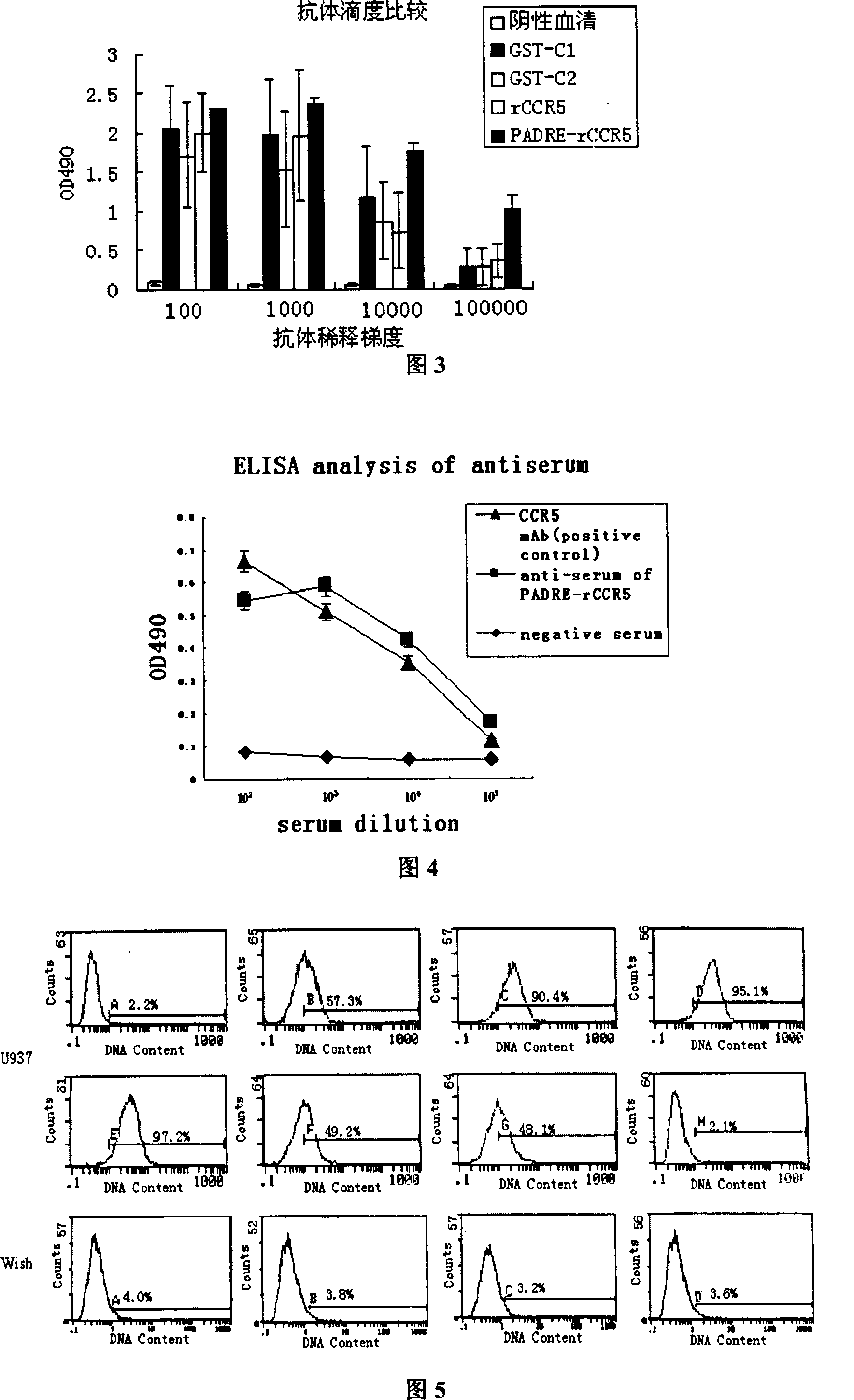
[0100] 1 Construction, expression and purification of CCR5 autologous peptide vaccine PADRE-rCCR5
[0101] 1.1 Construction of rCCR5 gene
[0102] Analyze the structure of CCR5 in the Swiss-Pro protein database, combine the literature, intercept the 4 extracellular N-TERM ECL1, ECL2, ECL3 amino acid sequences of CCR5, and use flexible linker to connect them in series to obtain the amino acid sequence of the analog CCR5 extracellular fragment (named: rCCR5), the four amino acid fragments outside the membrane of the receptor CCR5 and the Linker sequence are as follows:
[0103] N-TERM: MDYQVSSPIYDINYYTSEPCQKINVKQIAAR
(31aa)
ECL1: YAAAQWDFGNTMCQ(14aa)
ECL2: RSQKEGLHYTCSSHFPYSQYQFWKNFQTLK(30aa)
[0104] ECL3: NTFQEFFGLNNCSSSNRLDQAM(22aa)
Linker: GGGGS
[0105] 1.1.1 Synthesis of rCCR5 gene
[0106] According to the gene sequence of human CCR5 published by Gene-bank, the EcoRI restriction site is inserted at the 5 end, and the stop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com