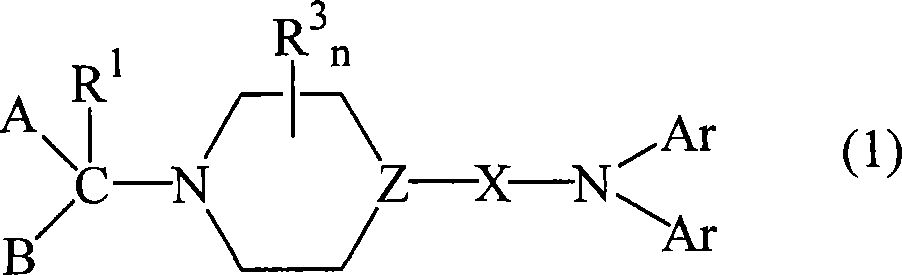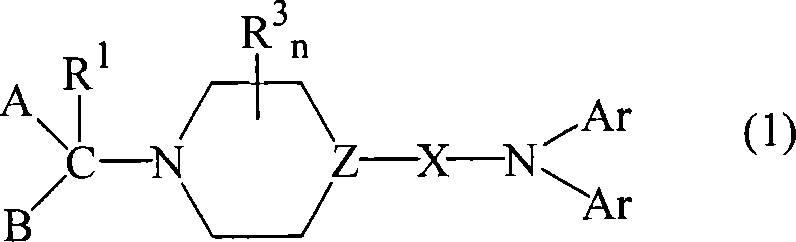Diarylamine derivatives as calcium channel blockers
A technology of diphenyl and diphenylamino, which is used in the field of compounds for the treatment of calcium channel function-related diseases, and can solve the problem of non-specificity of N-type channels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
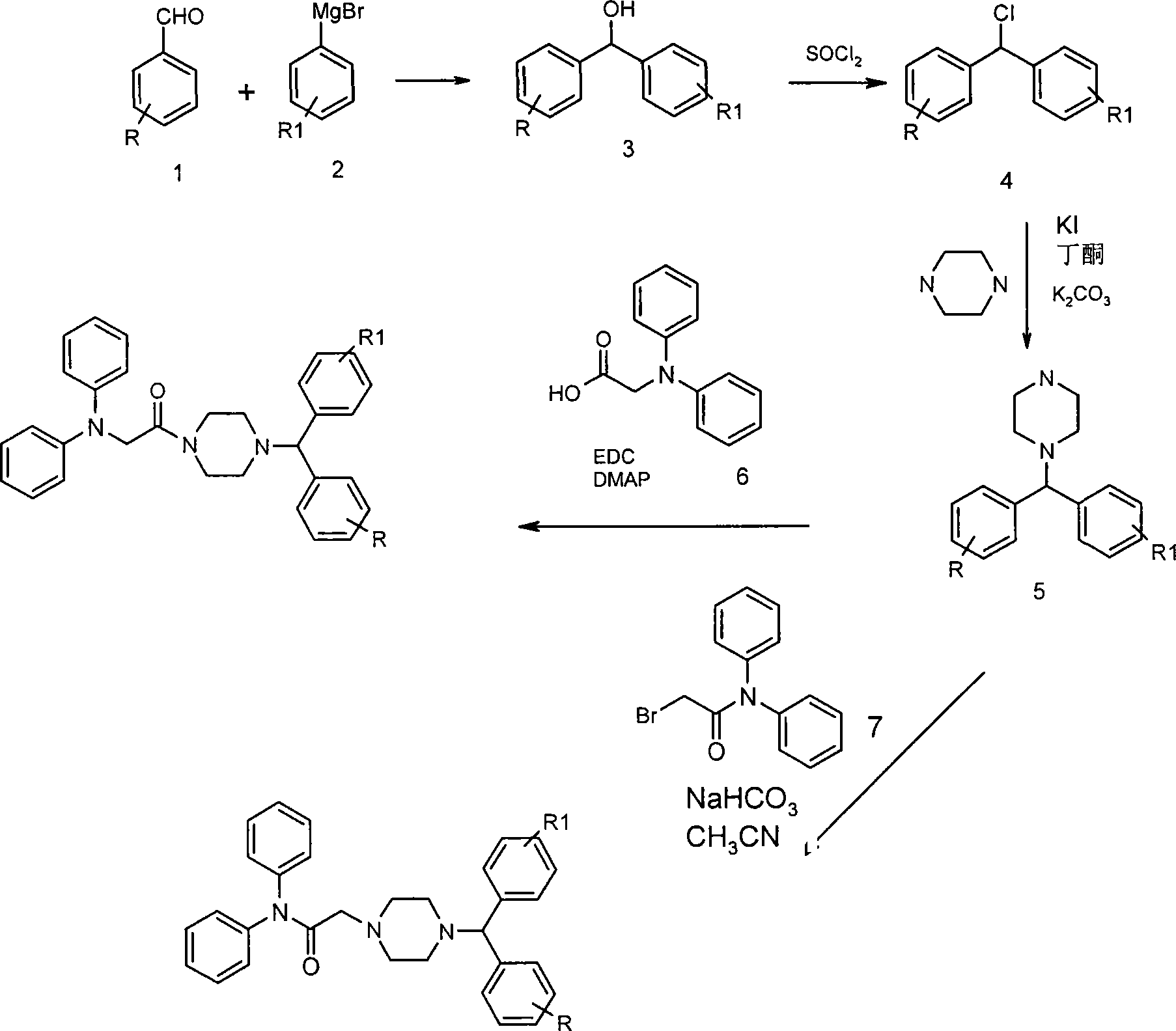
Embodiment 1
[0088] Synthesis of 1-{4-[(4-chloro-phenyl)-phenyl-methyl]-piperazin-1-yl}-2-diphenylaminoethanone
[0089]
[0090] A. Synthesis of (4-chloro-phenyl)-phenyl-methanol
[0091]
[0092] A solution of 4-chlorobenzaldehyde (1.03 g, 7.34 mmol) in anhydrous ether (10 mL) was added slowly to phenylmagnesium bromide (2.3 mL, 6.98 mmol, 3.0 M in ether) under nitrogen. The mixture was heated to reflux for 1 hour, then cooled to 0°C and hydrolyzed with 1N HCl (40ml). The aqueous phase was extracted with ether (3X), and MgSO 4 The combined organic layers were dried. The crude product was purified using hexane:ethyl acetate (5:1) as eluent to yield 1.5 g of pure product.
[0093] B. Synthesis of 1-chloro-4-(chloro-phenyl-methyl)-benzene
[0094]
[0095] SOCl 2 (8.25ml, 110mmol) and anhydrous CaCl 2 (2g) was added to a solution of (4-chloro-phenyl)-phenyl-methanol (2.41g, 11.06mmol) in anhydrous benzene (20ml). The mixture was heated to reflux for 2 hours, then coole...
Embodiment 2
[0102] Synthesis of 2-diphenylamino-1-[4-(phenyl-pyridin-4-yl-methyl)-piperazin-1-yl]-ethanone
[0103]
[0104] Diphenylglycine (0.51 g, 2.29 mmol) was added to 1-(phenyl-pyridin-4-yl-methyl)-piperazine (0.58 g, 2.29 mmol) in anhydrous CH under nitrogen. 2 Cl 2 (40ml) solution. EDC (0.878 g, 4.58 mmol) and DMAP (cat) were added to the reaction and the reaction mixture was stirred at room temperature overnight under nitrogen. The reaction was then concentrated under reduced pressure. The residue was dissolved in ethyl acetate:water (10:1) (150ml). The organic phase was washed with water (30ml, 2x) and 10% NaOH (30ml), washed with MgSO 4 Dry and evaporate to dryness. The resulting residue was purified by column chromatography with hexane:ethyl acetate (1:1) to give the desired product in 79% yield.
Embodiment 3
[0106] Synthesis of 2-(4-Benzhydryl-piperazin-1-yl)-N,N-diphenyl-acetamide
[0107]
[0108] 2-Bromo-N,N-diphenylacetamide (0.68g, 2.37mmol) and NaHCO 3 (0.4g, 4.74mmol) was added diphenylmethylpiperazine (0.6g, 2.37mmol) in anhydrous CH 3 CN (20ml) solution. The reaction mixture was refluxed overnight. After cooling, the solvent was evaporated, the residue was dissolved in water (15ml) and washed with CHCl 3 Extraction (3 x 50ml). with MgSO 4 The organic layer was dried and evaporated to dryness. The resulting residue was purified by column chromatography with hexane:ethyl acetate (2:1) to give the desired product in 84% yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com