Method for in vitro preconditioning of myoblasts before transplantation
a technology myoblasts, which is applied in the field of in vitro preconditioning of myoblasts before transplantation, can solve the problems that it is not possible in clinical studies to use damaging treatments such as marcaine, notexin and irradiation, and achieve the effect of increasing the migration distan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0033] Materials and Methods
[0034] Myoblast cultures
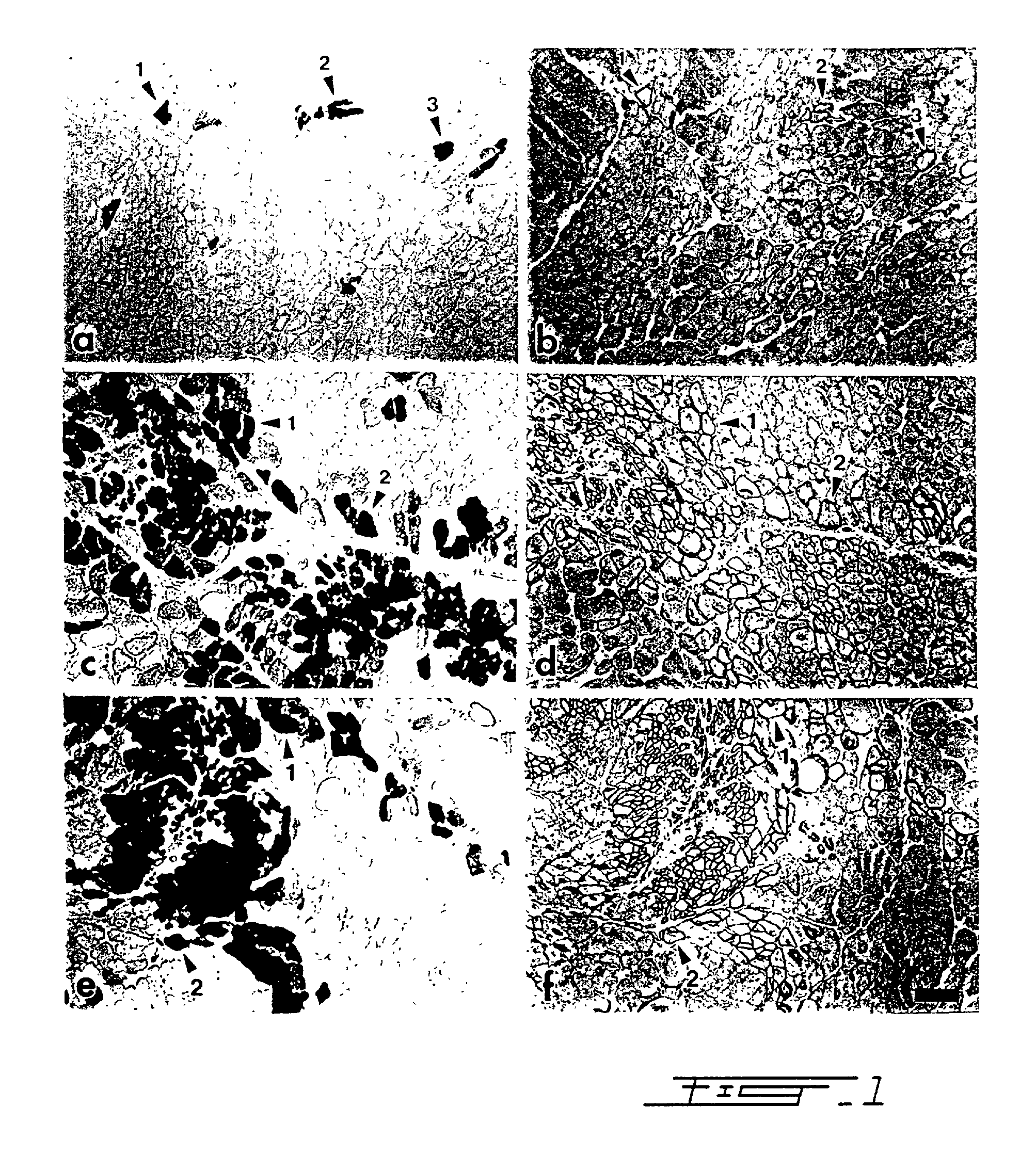
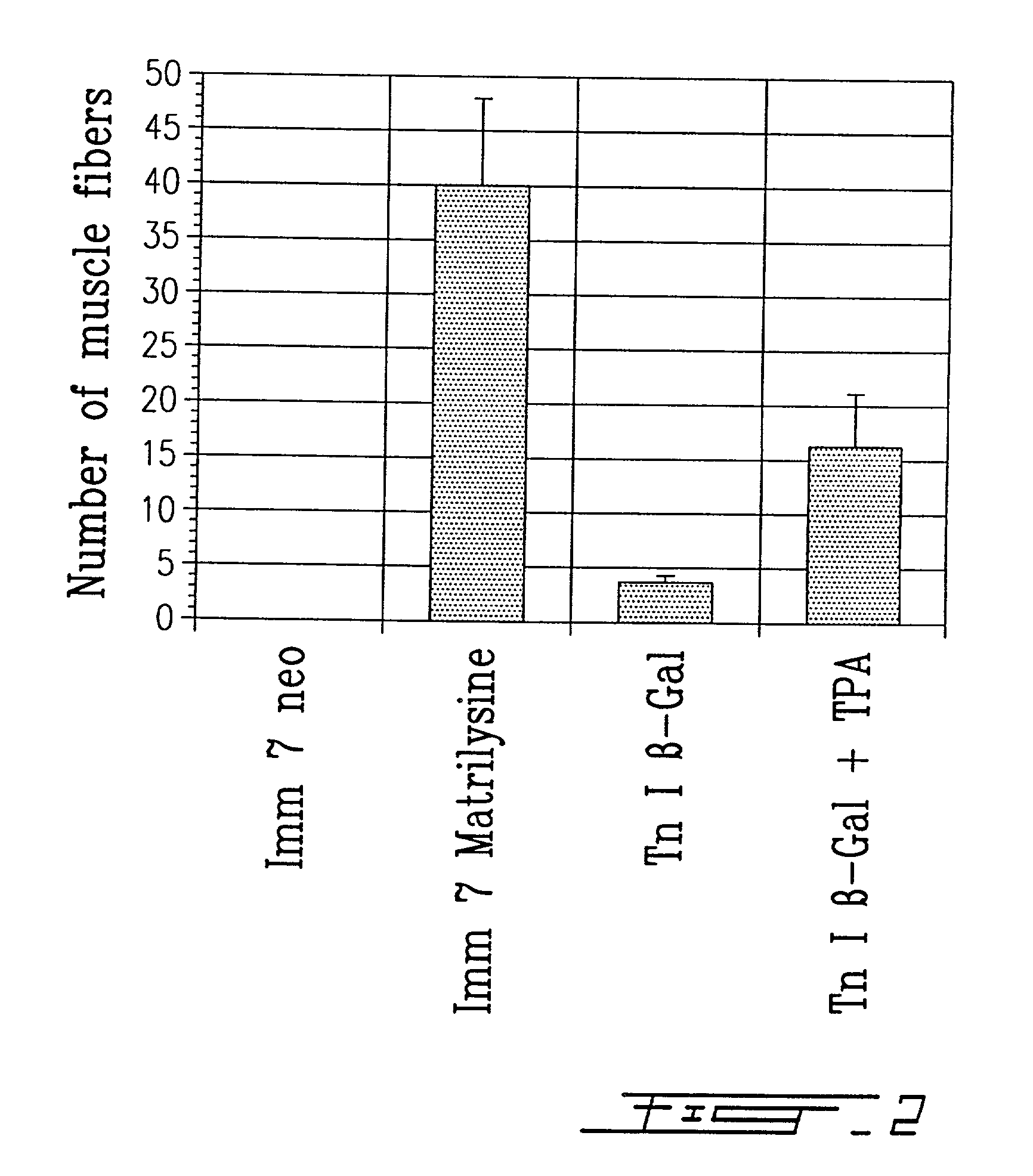
[0035] Primary myoblast cultures were established from muscle biopsies of newborn transgenic mice.sup.26. The founder mouse (TnI Lac Z1 / 29) was provided by Dr. Hasting (McGill University, Montreal, Canada) onto the CD1 background and was reproduced in our laboratory. This transgenic mouse expresses the .beta.-galactosidase gene under the control of the promoter of the quail fast skeletal muscle troponin I gene.sup.16. Blue muscle fibers are revealed in these transgenic mice following incubation with a substrate, 5-brom-4-chlor-3-indolyl-.beta.-D-galactopyronoside (X-gal) (Boehringer Mannheim Canada, Laval, Canada). Before starting myoblast cultures, it was necessary to identify transgenic newborns by X-gal staining of a small muscle biopsy because heterozygote transgenic mice were used as parents. Myogenic cells were released from skeletal muscle fragments of the transgenic newborns by serial enzyme treatments. First, a one hour di...
example 2
[0052] The above results can be extrapolated to an in vivo utility and verified in patients suffering of muscular dystrophy. The healthy donors and DMD recipients should be matched, if possible, upon their compatibility for the MHC (HLA)-class I (A, B, C) and-class II (Dr) antigens. The dystrophic patients should undertake an immunosuppressive treatment by being administered, for example, FK 506, cyclosporin, RS61443 or rapamycin. Donors' biopsy would then be treated substantially in accordance with the procedures given in Example 1 with regard to mice myoblasts. The success of the transplantation might be monitored by measuring the incidence of dystrophin-positive fibers from a biopsy obtained from the site of transplantation and by evaluating the resulting increase of muscular strength.sup.39.
example 3
[0053] Myoblasts infected with a retrovirus expressing a beta-galactosidase gene have been cultured for four days in the presence or absence of 20.mu.g / ml concanavalin A, a lectine which stimulates the expression of metalloproteases. These myoblasts were then injected in one single site of the anterior tibialis muscle of eight mice, in order to verify the degree with which the transplanted cells are capable of migrating through the recipient tissue. After thirty days, mice were sacrificed and the muscle tissue was harvested, frozen and 10 .mu.m thick slices were mounted on slides. The presence of beta-galactosidase was revealed with X-Gal. Labelled cells were observed at a distance which is 3 to 4 fold greater in the mice muscle treated with concanavalin A. Concanavalin A is known to induce the secretion of metalloproteases by the fibroblasts present in the primary cultures. Therefore, the presence of metalloproteases in the preconditioning medium or during the transplantation is be...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| thick | aaaaa | aaaaa |
| distance of migration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com