Improved immortalized human skin cell lines and novel serum-free medium useful for the production thereof
a technology of immortalized human skin and serum-free medium, which is applied in the direction of cell culture active agents, artificial cell constructs, genetically modified cells, etc., can solve the disadvantages of their use, achieve moderate effect on keratinocyte cell growth, improve both the attachment of keratinocytes, and enhance normal keratinocyte growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
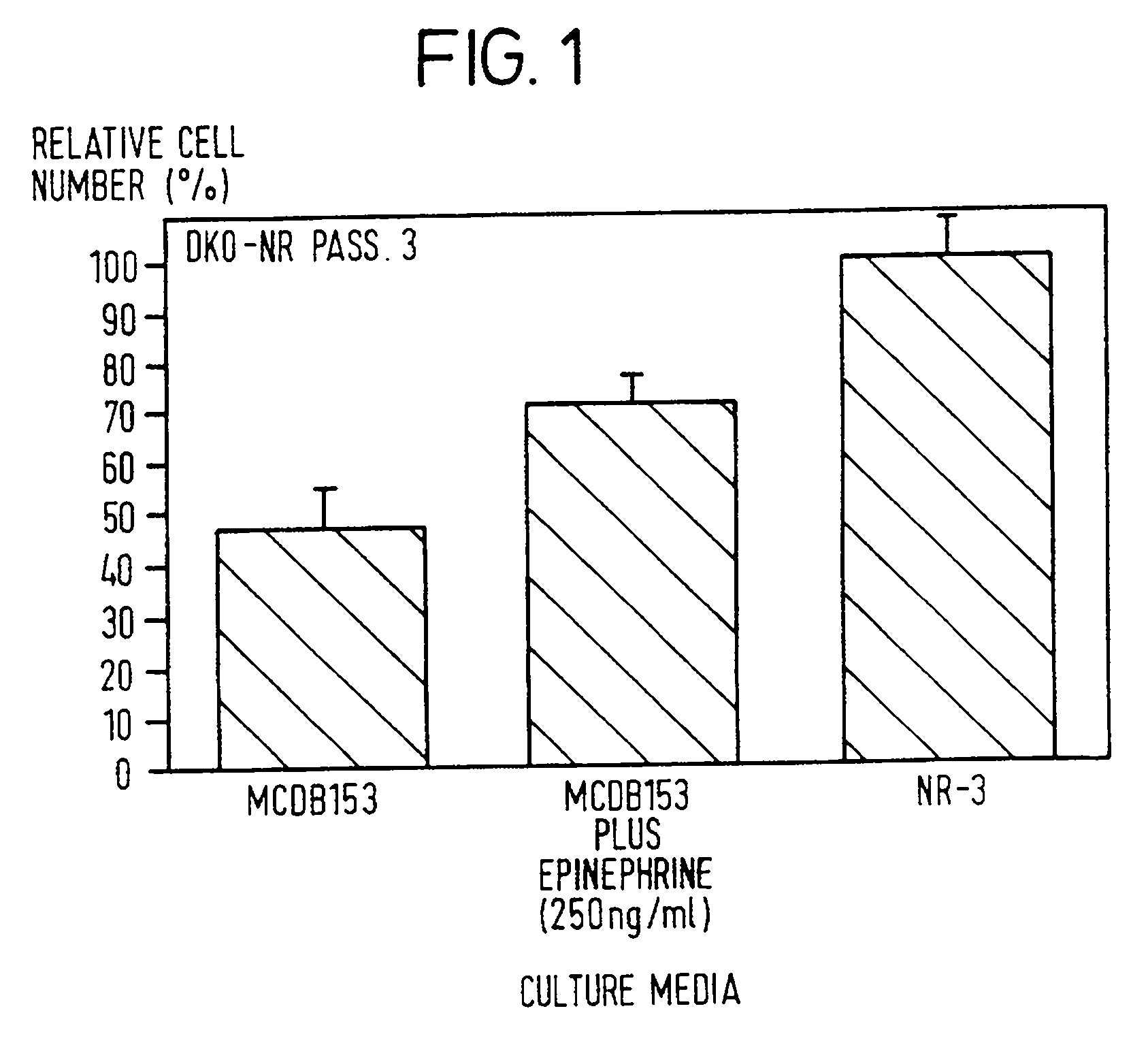
[0090] 1) Characterization of Keratinocytes Growth in Serum-free Media: primary cell cultures were cultivated in modified MCDB 153 [Boyce et al., J. Tissue Cult. Meth., 9:83-93 (1985); and Pittlekow et al., J. Invest. Dermatol., 86:410-417, 1986] and NR-3 media. The best cell growth has been observed in NR-3 medium (FIG. 1). Improved cell growth has been also observed in fully defined NR-3 medium (NR-3 without bovine pituitary extract, BPE) compared to modified MCDB 153 without BPE.
[0091] FIG. 1 comprises cell growth in NR-3 and epinephrine-supplemented modified MCDB 153 (keratinocyte growth medium) after 6 days. The modified MCDB 153 medium refers to modified MCDB 153. Keratinocytes were harvested in trypsin / EDTA (0.05% / 0.01%) and counted by using a hemocytometer. The results shown in FIG. 1 are the mean of triplicates.
[0092] 2) Effect of Coating on Cellular Attachment and Cell Growth: the coating of culture plates was found to improve the cell attachment and the cell growth of nor...
example 3
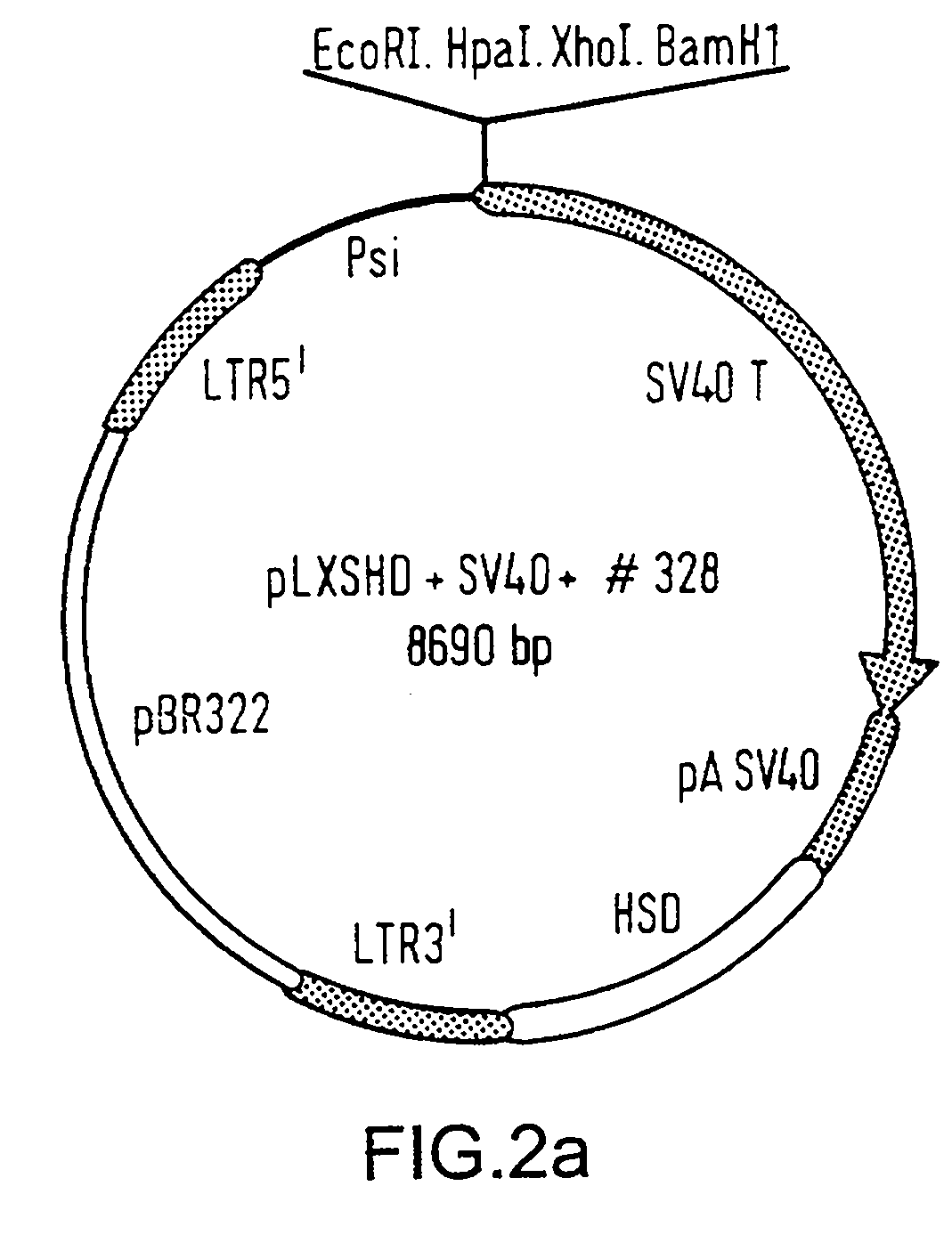
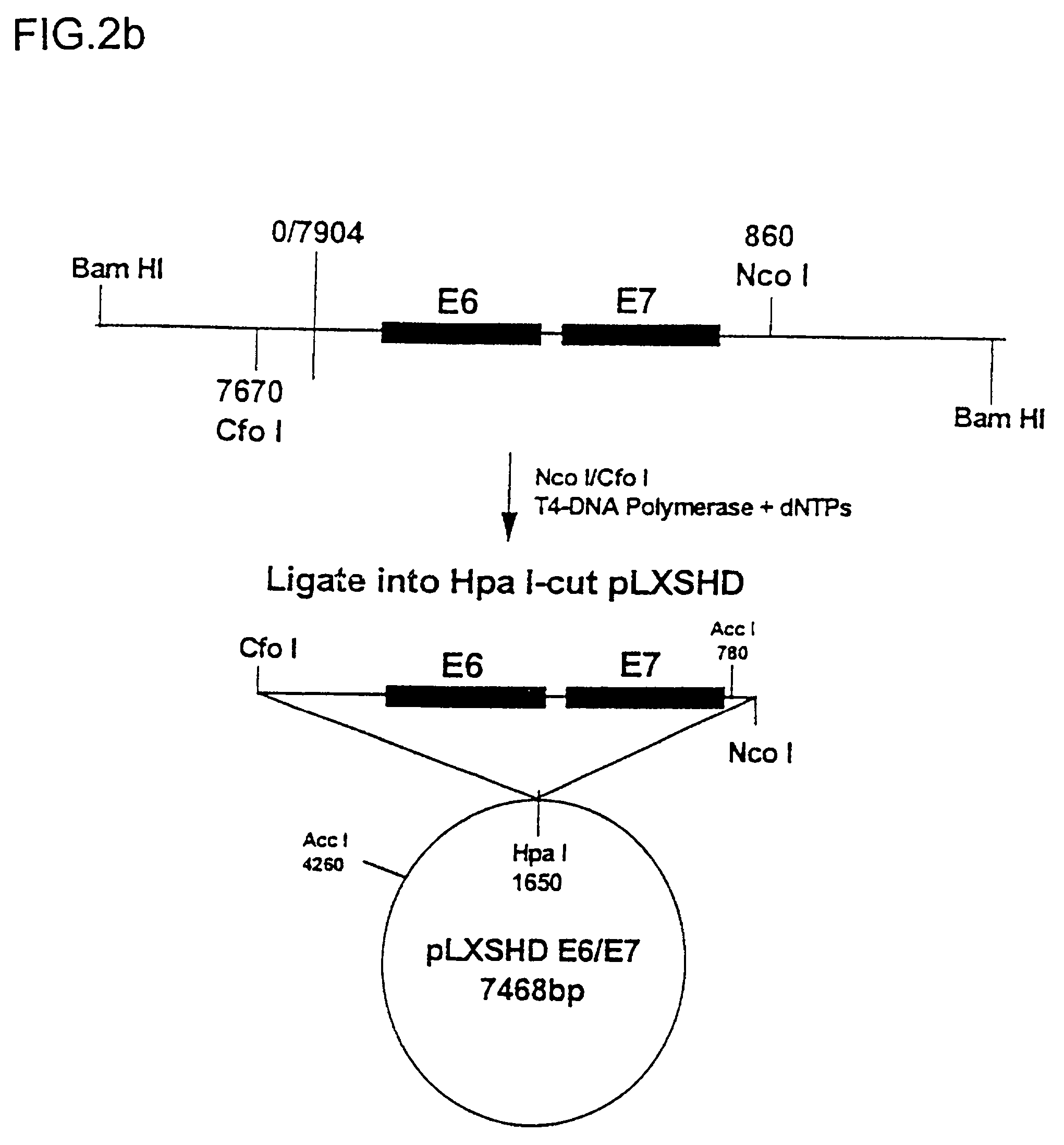
[0093] 1) Immortalization of Keratinocytes: a cell suspension produced from skin samples described in example 1, which contains dissociated melanocytes, keratinocytes and fibroblasts, are cultured in the subject NR-3 medium. This is effected by seeding such cells onto culture dishes which are continuously coated with the "cocktail" coating previously described for bronchial cells (Lechner et al., J. Tiss. Cult. Meth., 9:43-48 (1985)). During culturing the primary cell cultures when they reach or substantially reach confluence, the cells are treated for 4 min with trypsin / EDTA (0.025% / 0.01%). During this treatment, the melanocytes detached from the keratinocytes culture, and they are collected separatly. Primary melanocytes and keratinocytes are thus separated at this stage. After the primary keratinocytes have been cultured and expanded to desired cell numbers in NR-3 serum-free medium using the described coated culture dishes (promotes the growth of keratinocytes versus melanocytes...
example 4
[0108] 1) Immortalization of melanocytes: a cell suspension produced from skin sample DKO-NR described in example 1, which contains dissociated melanocytes, keratinocytes and fibroblasts, are cultured in the subject NR-3 medium. This is effected by seeding such cells onto culture dishes which are continuously coated with the "cocktail" coating previously described for bronchial cells (Lechner et al., J. Tiss. Cult. Meth., 9:43-48 (1985)). During culturing the primary cell cultures when they reach or substantially reach confluence, the cells are treated for 4 min with trypsin / EDTA (0.025% / 0.01%). During this treatment, the melanocytes detached from the keratinocytes culture, and they are collected separatly. Primary melanocytes and keratinocytes are thus separated at this stage. The collected primary melanocytes are then seeded in NR-4 serum-free medium which specifically inhibits the growth of keratinicytes. After the primary melanocytes have been cultured and expanded to desired ce...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| physical | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com