Procollagen (III) Propeptides and Related Substances for Treating Fibrotic Diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
Renaturation of recombinant collagen .alpha. 1(III) propeptides.
[0086] As an example, recombinant human PIIICP4.1 was renatured, the production of which was described in Burchardt, 1998, and in the patents: An immunoassay for procollagen-III-C-terminal propeptide (WO 99 / 24835A2 and EP00988964A1). Furthermore, recombinant human PIIINP 4.5.2 was renatured, the production of which was described in the patent: Monoclonal antibody and assay for detecting PIIINP (WO 99 / 61477A2). Finally, recombinant murine PIIINP, the production of which was described in Kauschke, 1999, was renatured using the method described below.
[0087] However, the method is not limited to the renaturation of these exemplary propeptides, but is suitable for the renaturation of other procollagen propeptides and similar compounds.
[0088] Method.
[0089] Used Buffers.
[0090] A) Dialysis Buffer.
[0091] 300 mM Trizma-Base, pH 7.4.
[0092] 400 mM L-Arginine.
[0093] 10 mM EDTA.
[0094] 0.4 mM Pefabloc SC.
[0095] 1 mM Glutathione reduce...
example 3
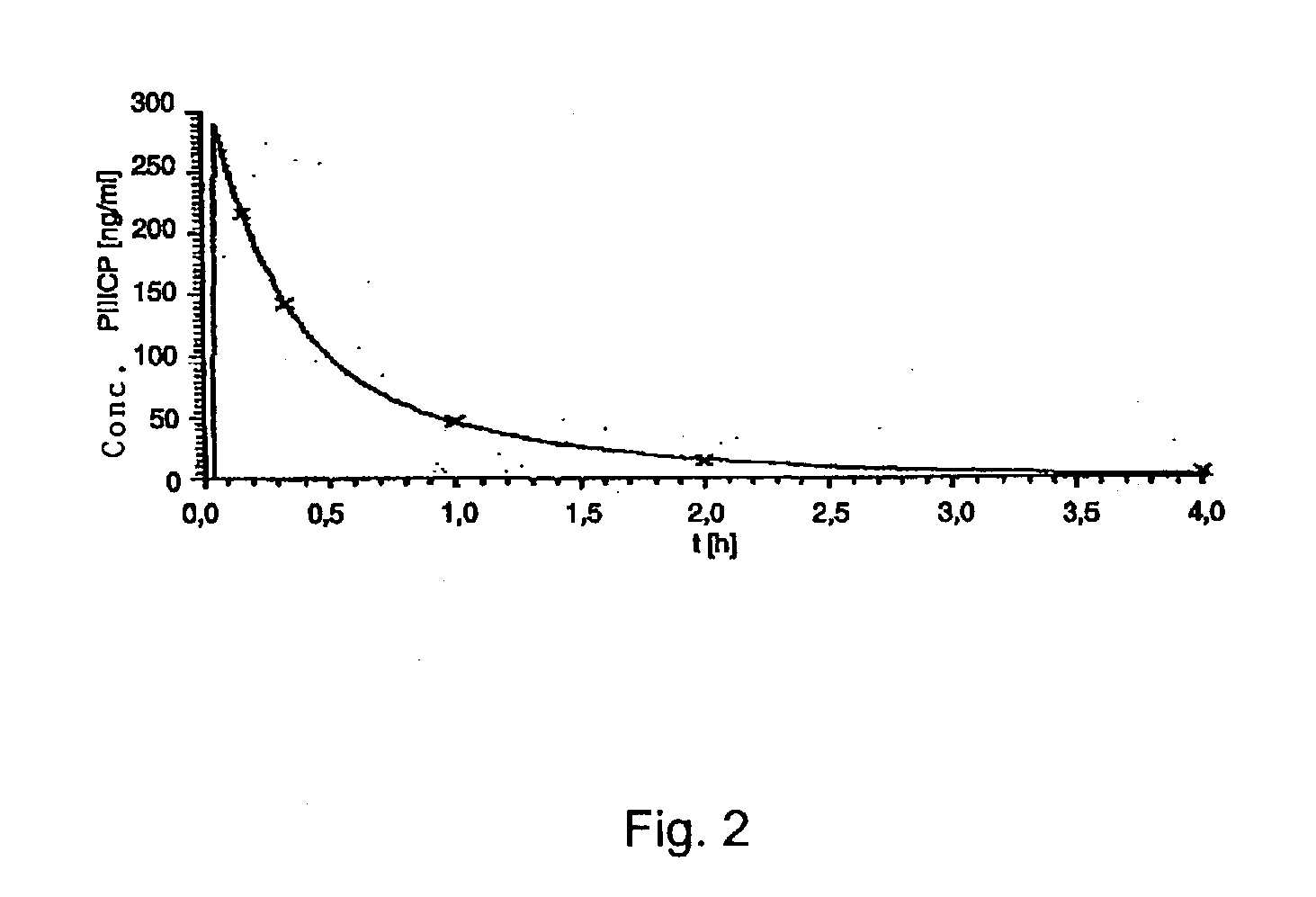
Measurement of the Elimination Kinetics of Renatured PIIICP in the Anaesthetized Rat.
[0107] Materials and Methods.
[0108] PIIICP-Plate-ELISA: The determination of PIIICP concentrations in biological samples was carried out with a sandwich ELISA assay. Two monoclonal anti-PIIICP antibodies were used (Burchardt, 1998).
[0109] As a catching antibody, antibody 48B14 (Burchardt, 1998) was immobilized on an ELISA plate at a concentration of 5 .mu.g / ml. After blocking free unspecific binding sites on the plate by incubation with a 3% (v / v) BSA solution, biological samples or buffered solutions with known PIIICP concentrations were added together with a known concentration of a FITC-labelled secondary antibody (48D19) (Burchardt, 1998) for 30 minutes to the immobilized secondary antibody. Remaining free PIIICP antigen and free secondary antibody were subsequently removed by washing steps and the amount of bound, labelled secondary antibody was determined. The aforementioned antibodies can be ...
example 4
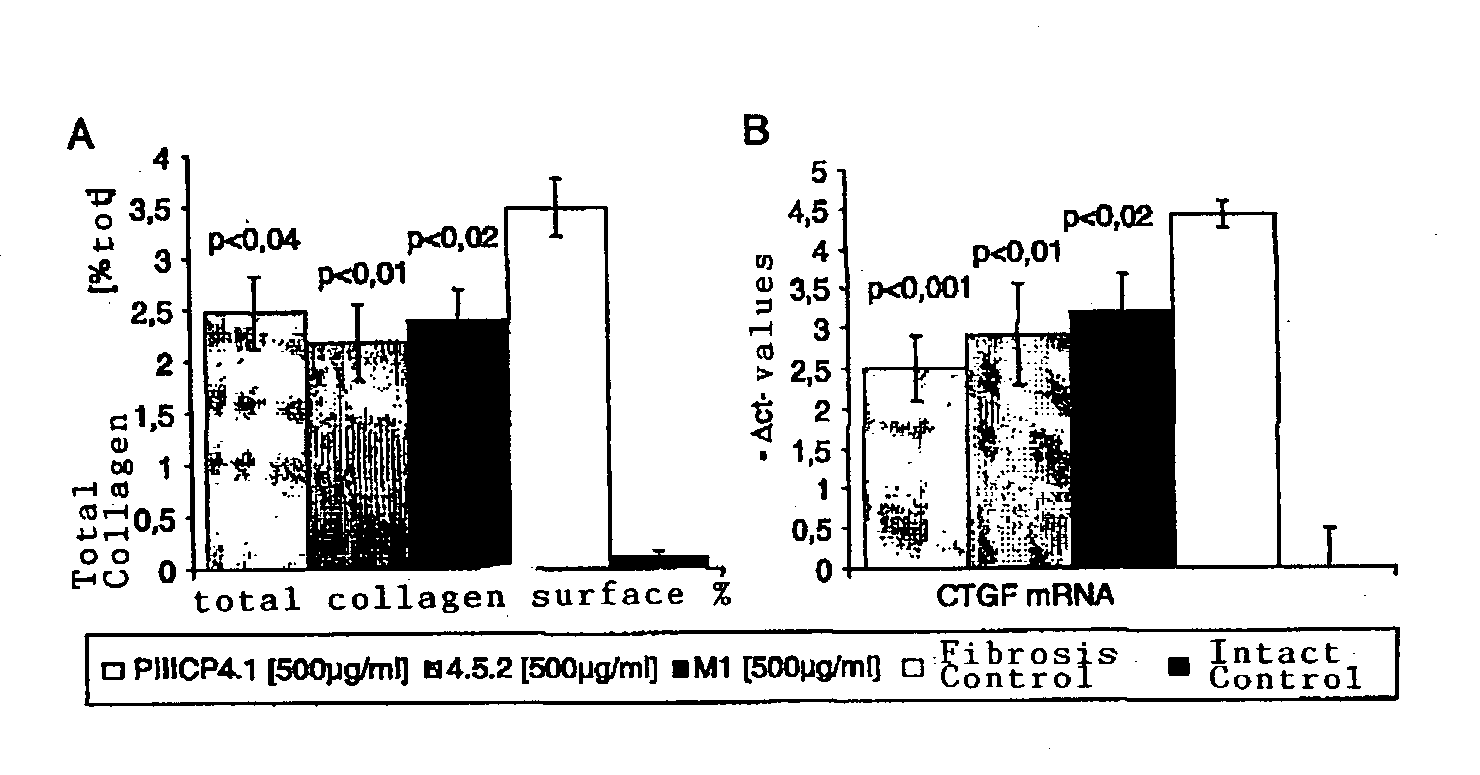
Demonstration of the Biological Efficacy of PIIICP and PIIINP.
[0115] The biological efficacy of the compounds can be demonstrated in cell culture assays and in vivo. For example, after addition of the inhibitors to human cell lines, a drop in the concentration of free .alpha. 1(III) propeptide in the supernatant can be measured because the peptide is released by the enzymatic activity of PCP. To measure PIIICP concentrations in the supernatant a recently established assay can be used (Burchardt, 1997, and patent application: An immunoassay for procollagen-III-C-terminal propeptide (WO 99 / 24835A2 and EP00988964A1)).
[0116] In this patent the biological efficacy of PIIICP4.1 (production described in Burchardt, 1998, and in the patents: An immunoassay for procollagen-III-C-terminal propeptide (WO 99 / 24835A2 and EP00988964A1), purification and renaturation described above in this patent as an example); of PIIINP4.5.2 (production described in the patent: Monoclonal antibody and assay for ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com