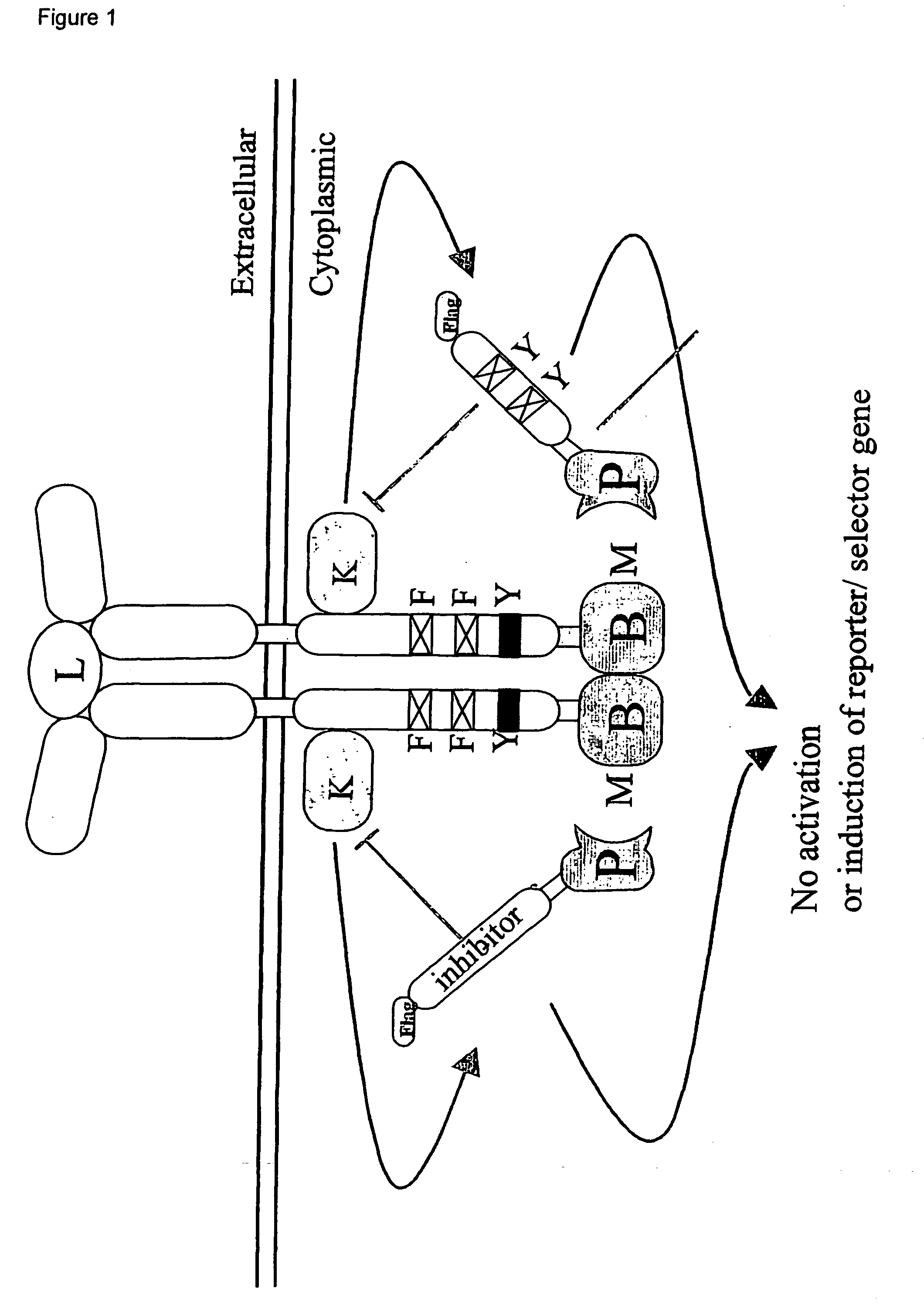
Reversed mammalian protein-protein interaction trap
a technology of protein and trap, which is applied in the field of reversed mammalian proteinprotein interaction trap, can solve problems such as inability to modify, and achieve the effects of facilitating identification and/or isolation, negative feedback, and optimizing the flexibility of proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
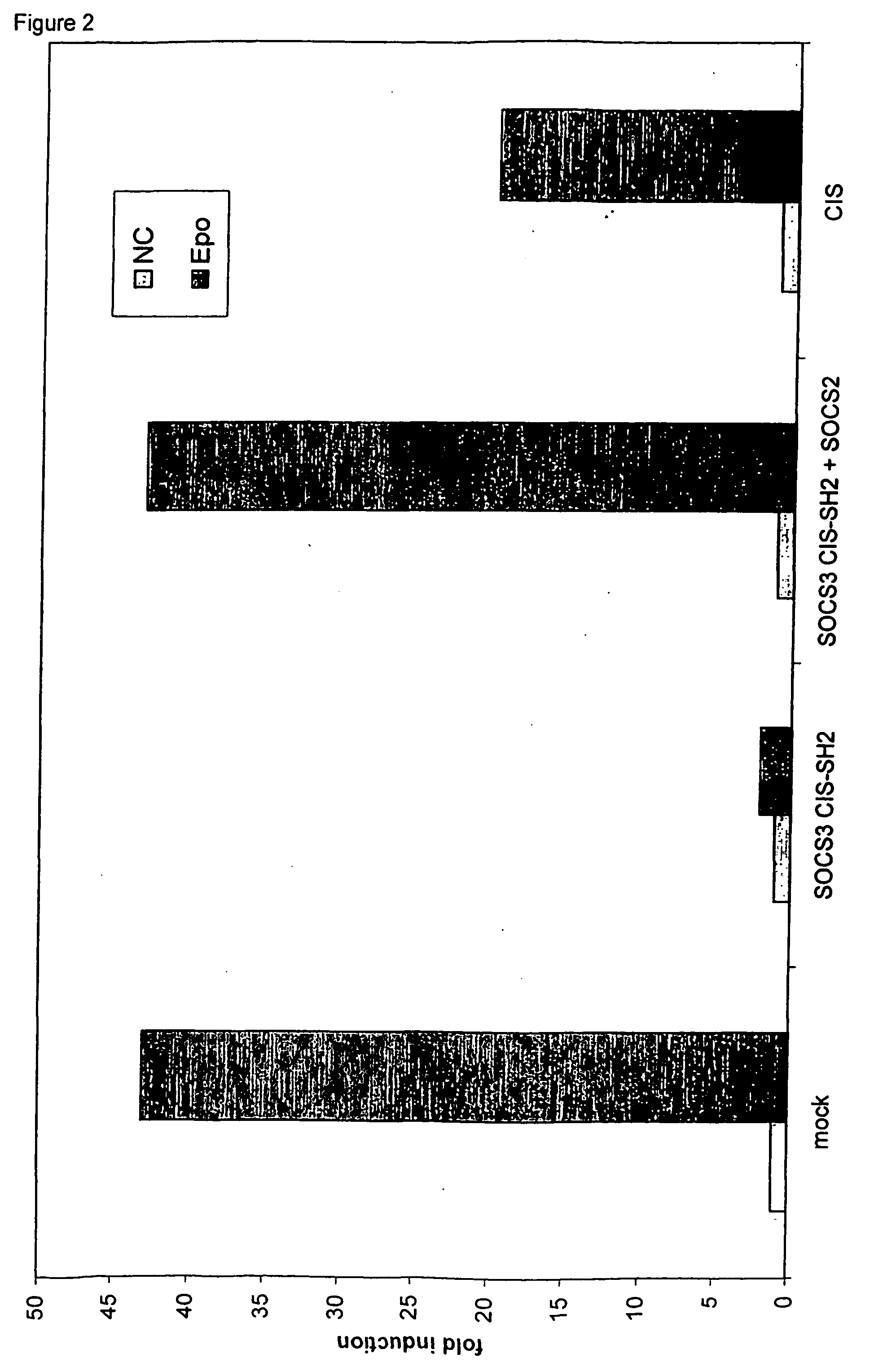
Specific Inhibition of Activation of the EpoR-LepRFFY-EpoR by the SOCS3 CISSH2 Chimera is Disrupted by Overexpression of SOCS2
[0110] The following combinations of plasmids were transfected in 4.times.10.sup.5 HEK293T cells.
[0111] a. pSV-EpoR-LepR FFY-EpoR+pMET7mcs+pXP2d2-rPAP1-luci+pUT651
[0112] b. pSV-EpoR-LepR FFY-EpoR+pMET7-fSOCS3 CISSH2+pXP2d2-rPAP1-luci+pUT-651
[0113] c. pSV-EpoR-LepR FFY-EpoR+pMET7-fSOCS3 CISSH2+pEF-FLAG-I / SOCS2+pXP2-d2-rPAP1-luci+pUT651
[0114] d. pSV-EpoR-LepR FFY-EpoR+pMET7-fCIS+pXP2d2-rPAP1-luci+pUT651
[0115] DNA amounts in a 300 .mu.l precipitation mixture were: 1 .mu.g pSV-EpoR-LepRFFY-EpoR chimeric receptor construct, 1 .mu.g of pXP2d2-rPAP1-luci reporter construct, 25 ng of pUT651 for normalization and 100 ng of the other plasmids. Additional pMET7mcs vector was added to keep DNA amounts constant in the transfections. 200 .mu.l of this precipitation mixture was added to 4.times.10.sup.5 HEK293T cells in 6-well plates. Twenty-four hours after transfection, c...
example 2
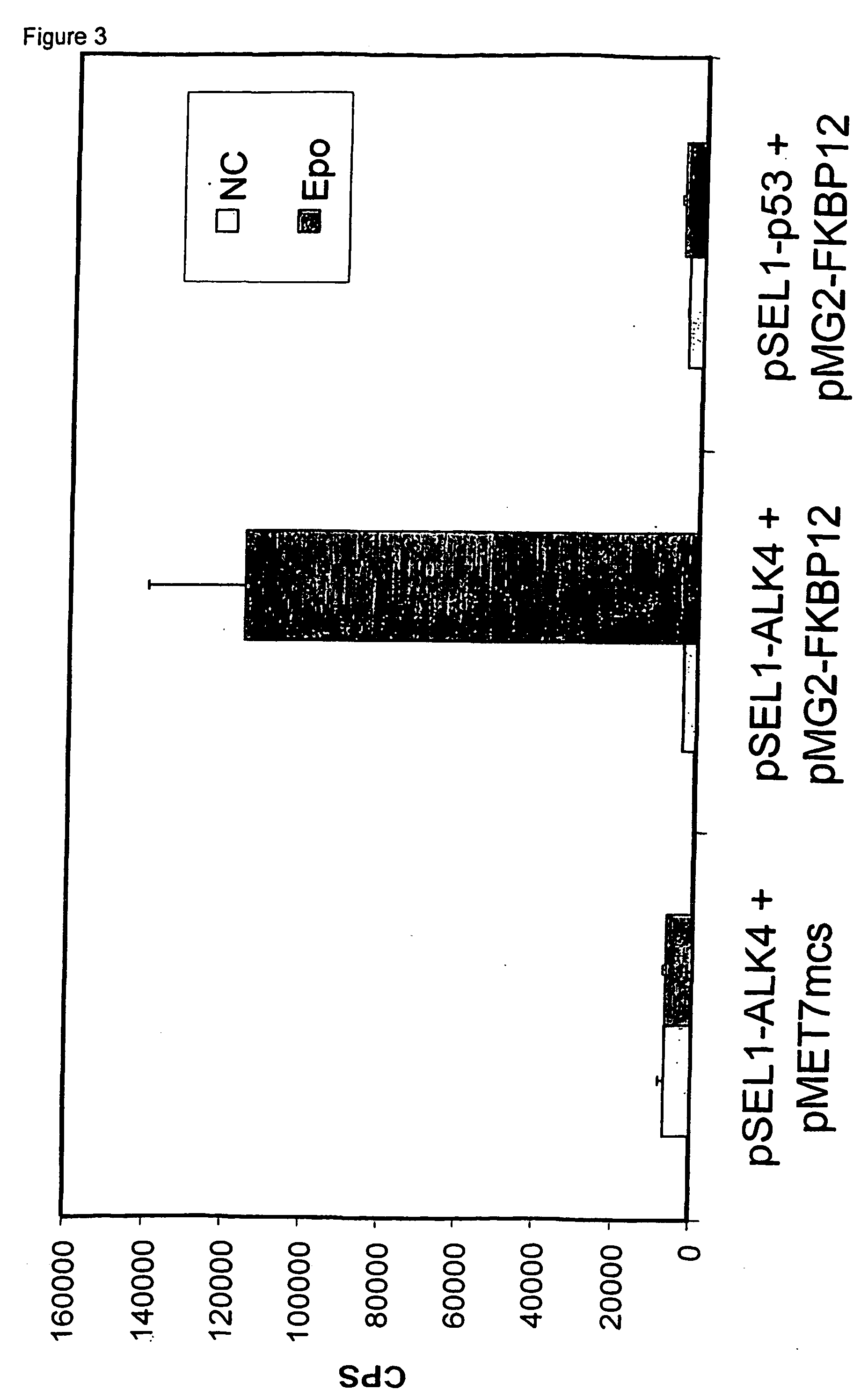
Disruption of the ALK4-FKB12 Interaction by FK506
[0117] To demonstrate the feasibility of the technique, a normal ALK4-FKB12 interaction, measured with a positive read-out receptor-based interaction trap (i.e., induction of the luciferase activity, by activation of a recombinant receptor comprising a ligand-binding domain and a cytoplasmic domain that comprises a heterologous bait polypeptide, whereby the receptor is activated by binding of a ligand to the ligand-binding domain and by binding of a prey polypeptide, fused to an activation domain to the heterologous bait peptide, as described in the European patent application 00201771.3) was inhibited by the addition of FK506.
[0118] The ALK4-FKBP12 interaction has been described by Wang et al. (1994).
[0119] To test the ALK4-FKBP12 interaction in the positive read-out receptor-based interaction trap, 400,000 HEK293T cells (6-well plate) with a combination of the following plasmids:
[0120] 1. pSEL1-ALK4 (1 .mu.g)+pMET7mcs (1 .mu.g)+pXP2...
example 3
Expression of the pMET7-fPPD1-FKBP 12 Inhibitory Prey Leads to Inhibition of Signaling via the pSELFFY-ALK4 Bait and can be Blocked by FK506
[0127] In a first experiment, it was demonstrated that the signaling activity of pSELFFY bait constructs can be inhibited by the inhibitory prey pMET7-fPPD1-FKBP12, comprising the PTP-1B phosphatase domain. 400,000 HEK293T cells (6-well plate) were transfected with a combination of following plasmids:
[0128] 1. pSELFFY-ALK4 (1 .mu.g)+pMET7mcs (1 .mu.g)+pXP2d2-rPAP1-luci (200 ng)+pUT651 (25 ng)
[0129] 2. pSELFFY-ALK4 (1 .mu.g)+pMET7-fPPD1-FKBP12 (500 ng)+pMET7mcs (500 ng)+pXP2d2-rPAP1-luci (200 ng)+pUT651 (25 ng)
[0130] 3. pSELFFY-EpoR (1 .mu.g)+pMET7mcs (1 .mu.g)+pXP2d2-rPAP1-luci (200 ng)+pUT651 (25 ng)
[0131] 4. pSELFFY-EpoR (1 .mu.g)+pMET7-fPPD1-FKBP12 (500 ng)+pMET7mcs (500 ng)+pXP2d2-rPAP1-luci (200 ng)+pUT651 (25 ng)
[0132] pMET7mcs was added as indifferent plasmid to transfect each time with an equal amount of DNA.
[0133] Two days after transfe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Interaction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com