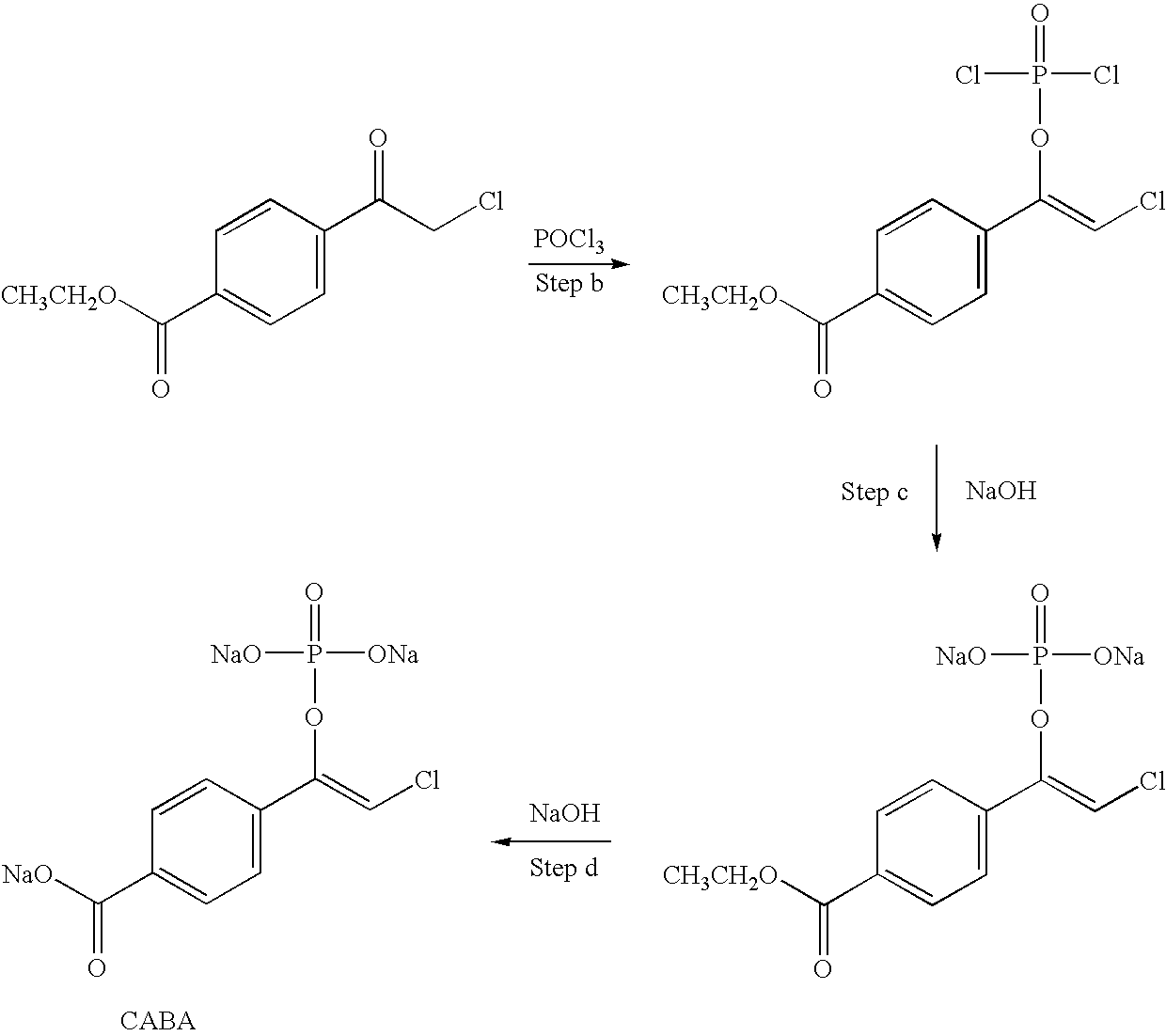
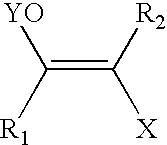
Protecting groups for biological labeling
a technology of biological labeling and protection groups, applied in the direction of phosphorous compound active ingredients, bulk chemical production, instruments, etc., can solve the problems of inability to store for extended periods of time in aqueous solutions, hydrolytic instability, and inapplicability to us
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Demonstration of Deprotection of the Alkylating Group
[0194] 1. Deprotection of a Phosphate Protecting Group by Alkaline Phosphatase
[0195] A mixture of BABA-phosphate and CABA-phosphate (resulting from preparation of BABA-phosphate), 5 mg in 1 mL pH 8-9 NaOD / D.sub.2O is treated with 1 mg (15 units) of alkaline phosphatase (Sigma, human placenta) for 15 minutes at room temperature. TLC analysis (250 micron SiO.sub.2 (Analtech Uniplate), using 20% H.sub.2O / ACN indicates that the phosphate group of the protected alkylating reagent is removed by treatment with alkaline phosphatase, whereas the nontreated protected alkylating reagent remains intact. This is further corroborated by NMR (D.sub.2O) analysis, which shows the disappearance of the vinyl proton peak (normally found at 6.3 ppm for the enol-phosphate).
[0196] Further demonstration of the efficient removal of the protecting group is shown by TLC (as above) analysis of the reaction of the deprotected alkylating reagent with cysteine....
example 2
Homocysteine Assay Using LOCI (Luminescent Oxygen Channeling Immunoassay)
[0199] A variety of assay methods and compositions are useful in the detection of compounds and compositions in biological samples--e.g., serum samples. One useful assay which uses the compositions and methods described herein is referred to herein as LOCI. (See U.S. Pat. Nos. 5,709,994, 5,340,716 and 5,478,729--the disclosures of which are incorporated by reference herein--for further details regarding luminescent oxygen channeling immunoassays.)
[0200] Hcy exists in numerous forms in plasma--e.g., as a small percentage in the free form, some as disulfides with itself and cysteine, and some as disulfides with albumin (about 70%). Total hcy is the clinically-relevant measure, however, with reference values in fasting subjects of about 5 to 15 .mu.mol / L. Even a moderate increase in plasma hcy, referred to as hyperhomocysteinemia, is considered a risk factor for premature cardiovascular diseases in the general pop...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com