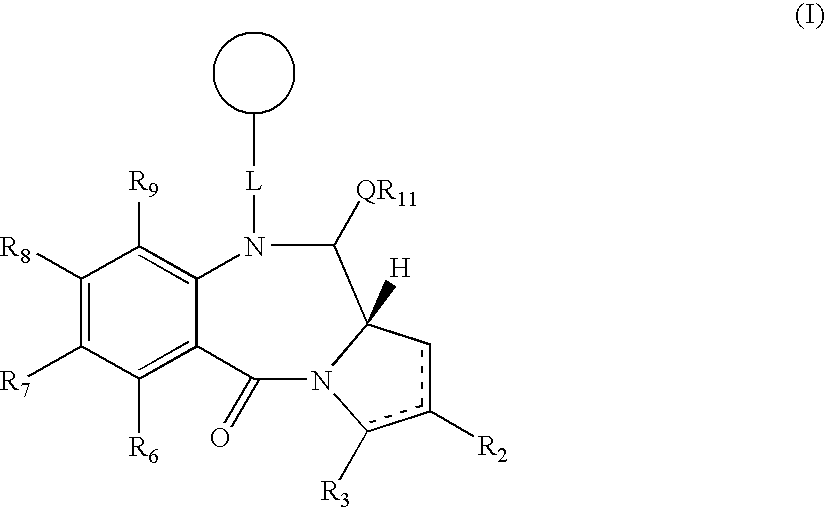
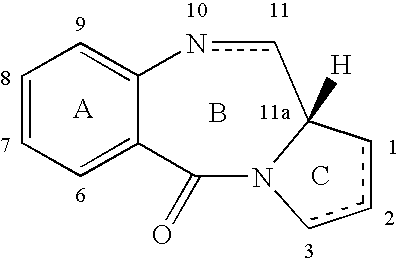
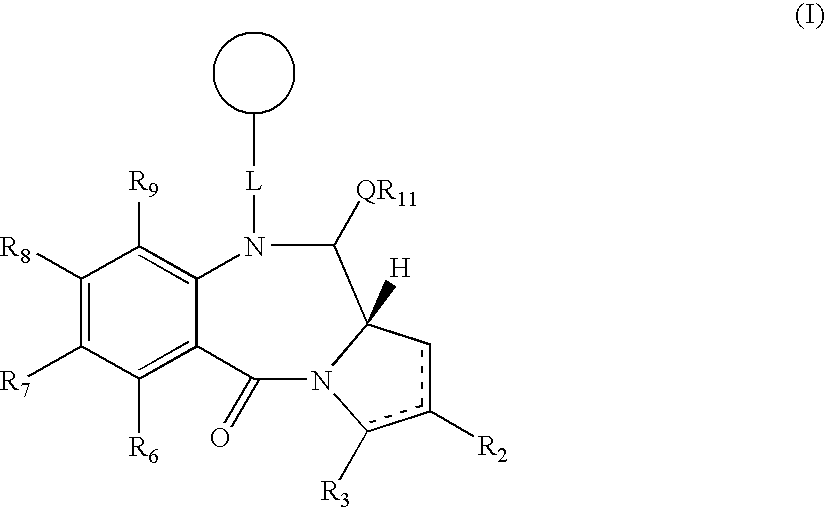
Collections of compounds
a technology of compounds and compounds, applied in the field of compounds, can solve the problems of unsuitability of techniques, less compatible with the range of possible c, and higher yield of
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
Synthesis of Resin-bound 7,8-Dimethoxy PBD (12) (FIG. 2)
[0116] Attaching Dimethoxy A-Ring to form Resin (10)
[0117] Dichloromethane CH.sub.2Cl.sub.2 (2 mL) was added to the choloroformate resin 2 (0.05 mmol) (prepared as in Example 1) and the vessel allowed to shake for 30 minutes. The suspension was cooled to 0.degree. C., a solution of 4,5-dimethoxyanthranilic acid 9 (0.05 g, 0.25 mmol) and pyridine (20 .mu.L) in NMP (2 mL) added, and the vessel allowed to shake at room temperature for 16 hours. The resin 10 was collected by filtration, and then rinsed with CH.sub.2Cl.sub.2 (2.times.5 mL), NMP (2.times.5 mL) and MeOH (2.times.5 mL). The entire procedure was repeated twice and the resin was then dried in vacuo. IR (reflectance, cm.sup.-1): 1750-1650 (CONH).
[0118] Attaching Pyrrolo C-Ring to form Resin (11)
[0119] Dimethyl formamide DMF (5 mL) was added to resin 10 (0.05 mmol) and the vessel allowed to shake for 30 minutes. Pyrrolidine methanol 5 (0.025 g, 0.25 mmol), TBTU (0.08 g, 0....
example 3
Alternative Synthesis of Resin-bound 7,8-Dimethoxy PBD (21) (FIGS. 3 & 4)
[0122] Overall Synthetic Strategy
[0123] The on-bead oxidation step employed in the previous approaches can be avoided by coupling an anthranilic acid loaded resin to the dimethyl acetal 16 derived from proline (FIG. 4). In this approach, unmasking of the dimethyl acetal protected aldehyde leads to spontaneous B-ring closure. Thus, exposure of the acetal 20 to a palladium catalyst (Pd(CH.sub.3CN).sub.2Cl.sub.2) leads to the formation of the cyclized compound 21. The acetal 20 was derived from the anthranilic acid resin 19 and the acetal 16, which were coupled together under standard conditions. The acetal 16 was obtained from the Cbz protected compound 15 (FIG. 3) by hydrogenation; i5 was in turn prepared by acetalisation of the aldehyde 14. Swern oxidation of the primary alcohol 13 afforded the aldehyde 14, the primary alcohol was prepared by a lithium tetrahydroborate reduction of the commercially available Cb...
example 4
Further Alternative Synthesis of 7,8-Dimethoxy PBD (13) (FIG. 5)
[0139] Overall Synthetic Strategy
[0140] This synthesis used the on-bead oxidation step of example 1 and 2 to obtain the resin-bound PBD 34, but using the Dess Martin reagent and Swern oxidation. The resin used to bind the required anthranilic acid 31 is p-nitrophenyl carbonate Wang resin, which can directly couple the anthranilic acid without the need for interactive transformation to the chloroformate and thus eliminating a process step.
[0141] Synthesis Route
[0142] A suspension of p-nitrophenyl carbamate Wang resin 31 (1 g, 0.93 mmol / g loading) in CH.sub.2Cl.sub.2 / DMF (2:1, 10 mL) was shaken for 30 minutes. A solution of dimethoxyanthranilic acid 9 (0.92 g, 4.7 mmol), HOBt (0.37 g, 2.8 mmol) and DIPEA (0.97 mL, 5.5 mmol) in CH.sub.2Cl.sub.2 / DMF (2:1, 20 mL) was added to the swollen resin. The vessel was allowed to shake at room temperature for 6 hours. Resin 32 was filtered and rinsed with DMF (2.times.10 mL), CH.sub.2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com