Compositions and methods for treatment of neurological disorders
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Beta Subunit TM2 Mutations Promote Agonist-Induced Residual Inhibition by ACh and Nicotine
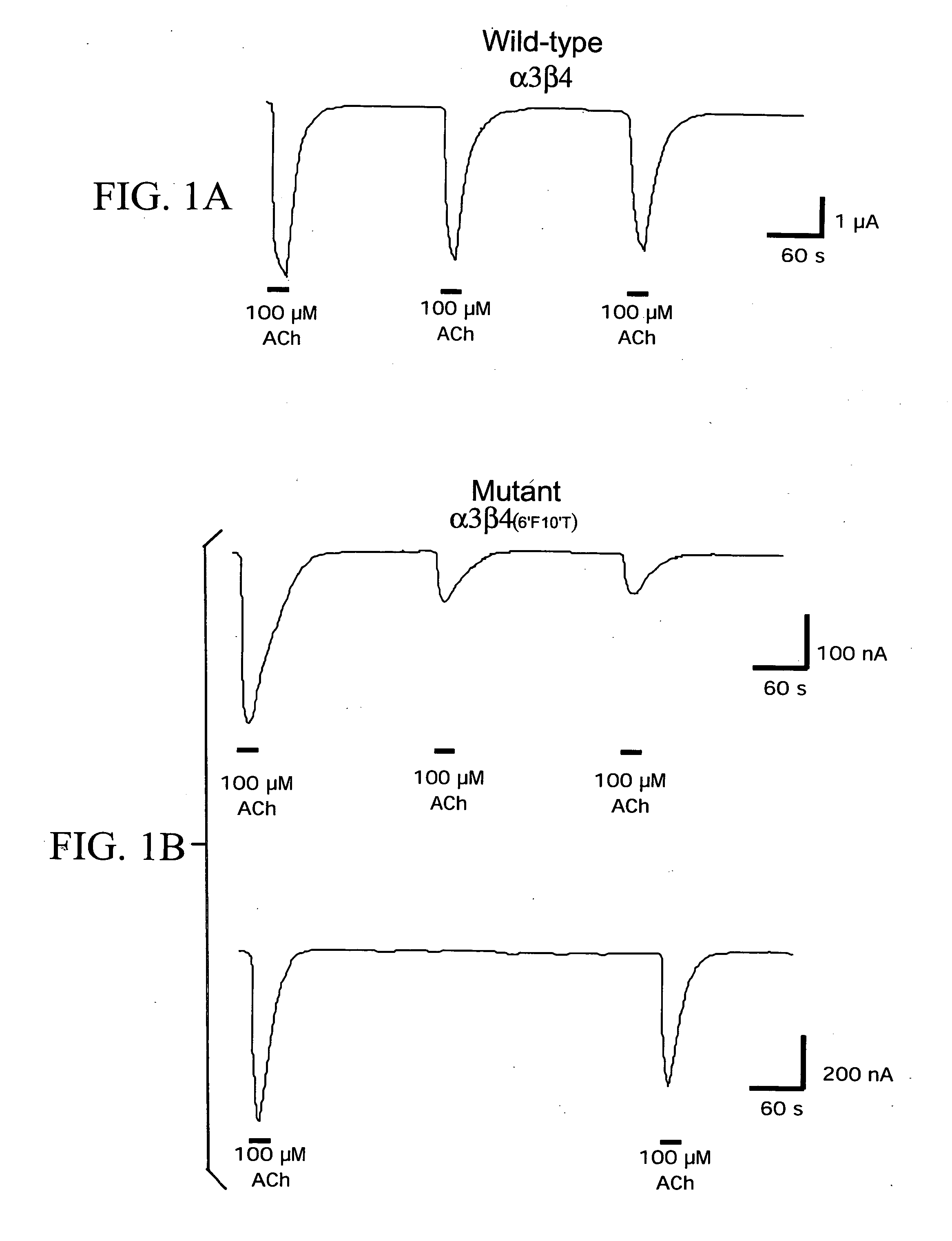
[0062] As previously reported for receptors containing chimeric β4 subunits (Webster, J. C. et al., Br. J. of Pharmacol. [1999] 127:1337-1348), receptors containing the β4 6′ and 10′ point mutations showed decreased responses to repeated applications of ACh and nicotine, suggesting that these agonists produce some form of residual inhibition, as shown in FIGS. 1A and 1B. While the inhibition produced by nicotine persisted for up to an hour (not shown), inhibition produced by ACh was more reversible, with essentially full recovery after 7-8 minutes of wash.
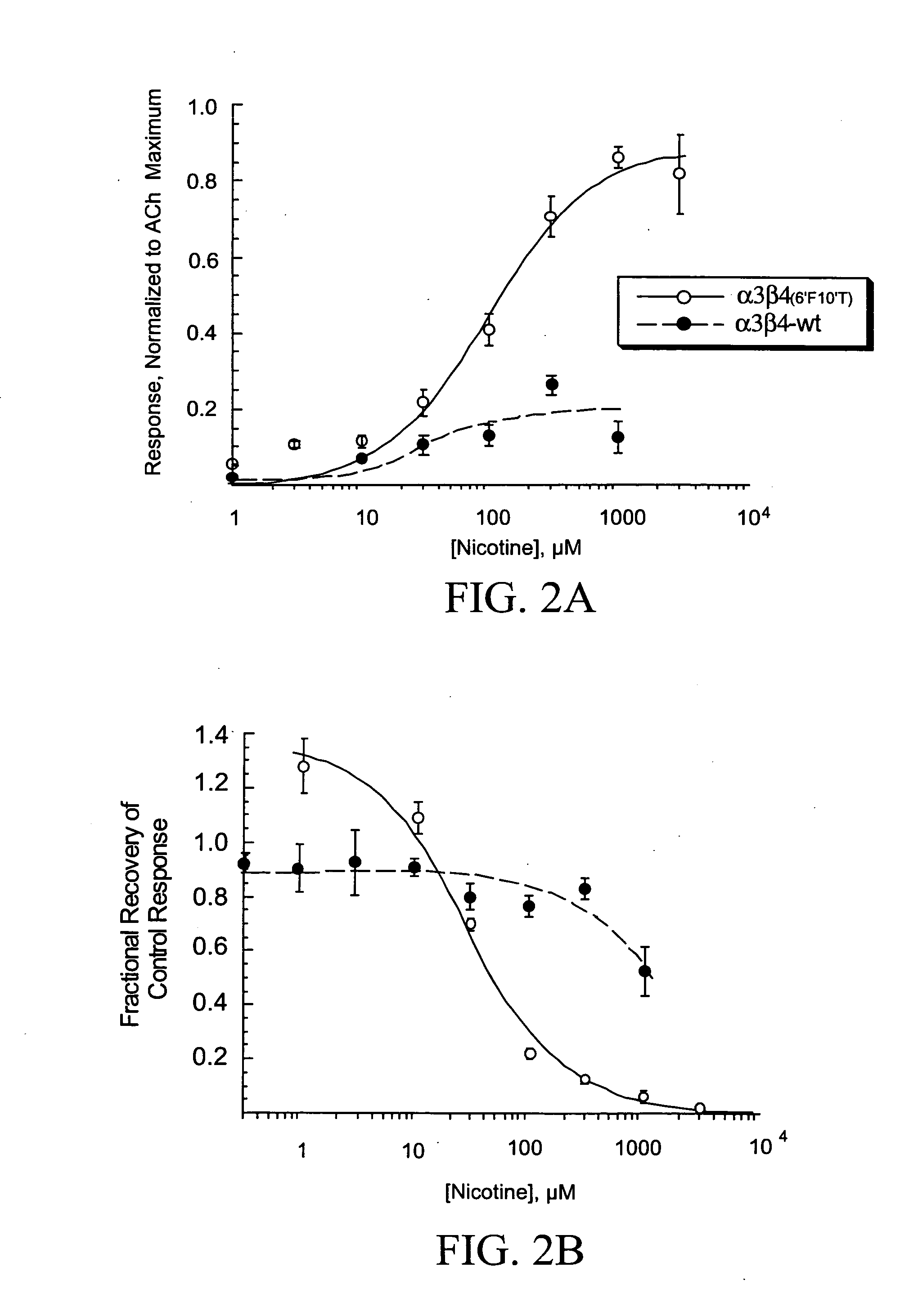
[0063] The concentration-response function for nicotine's activation and inhibition of wild-type and mutant receptors is shown in FIGS. 2A and 2B. The presence of the 6′ and 10′ mutations in the β4 subunit appeared to increase the efficacy of nicotine compared to ACh and to substantially increase the agonist-induced residual inhibition measu...
example 2
TM2 Mutations Differentially Regulate the Activation and Inhibition of Subtype-Selective Agonists
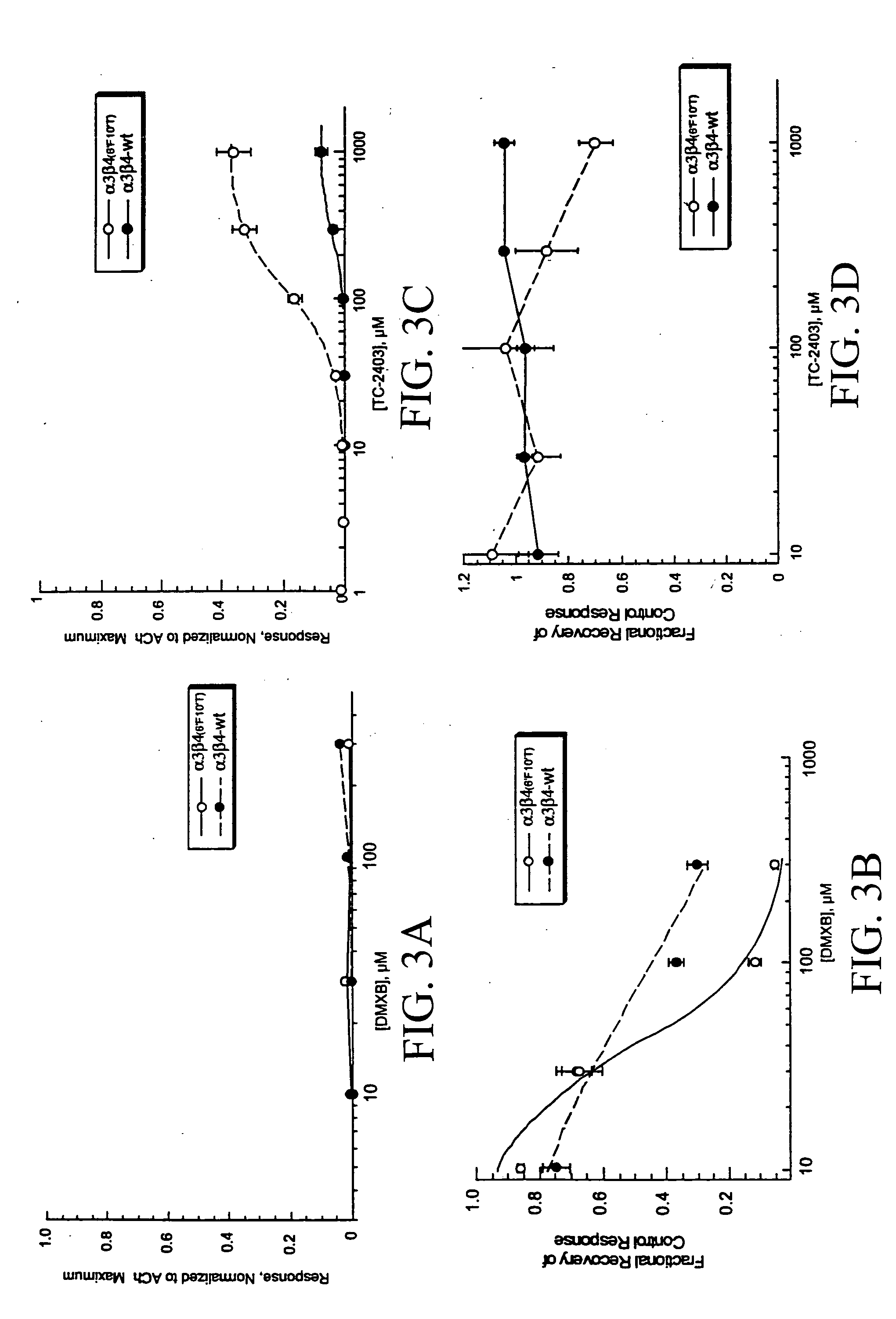
[0064] As shown in FIGS. 2A and 2B, the 6′ / 10′ mutations appear to influence both activation and agonist-induced residual inhibition, raising the question of whether these effects are likely to represent multiple consequences of these agonists binding to a single site on the receptor (i.e. the activation binding site), or alternatively represent effects from binding to multiple sites on the receptors. In order to test this, the effects of other agonists on the wild-type and mutant receptors were investigated. Specifically, two subtype-selective agents were used that previously have been reported to be only weak partial agonists on wild-type α3β4 receptors, DMXB and TC-2403 (metanicotine). DMXB is an α7-selective partial agonist (Meyer, E. M. et al., Brain Res. [1997] 768:49-56) that can produce agonist-induced residual inhibition of wild-type receptors in the absence of strong activatio...
example 3
The Residual Inhibition Produced by Agonists is Voltage Dependent
[0066] The voltage dependence of the residual inhibition of wild-type α3β4 and α3β4(6′F10′T) receptors produced by DMXB was evaluated, as well as the enhanced inhibition of α3β4(6′F10′T) mutant receptors by nicotine and ACh to see if the inhibition had properties that would be consistent with open channel blockade. Cells were held at either −50 mV or −100 mV and tested for their response to control concentrations of ACh. After a 10 min wash, test agonists (ACh, DMXB or nicotine) were applied at the concentrations indicated. Cells were then washed for 5 min and tested again for their response to a control ACh application. Cells were held at the indicated holding potential throughout the entire procedure. As shown in FIGS. 4A and 4B, the residual inhibition of both wild-type and mutant receptors was enhanced if the cells were held at a hyperpolarized potential. This would be consistent with inhibition associated with bi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Antagonistic activity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com