Catalyst composition and method for oxidizing mixtures
a technology of catalyst mixture and composition, which is applied in the direction of organic compound/hydride/coordination complex catalyst, physical/chemical process catalyst, chemical catalyst, etc., can solve the problem of proportions of catalyst mixture falling out of suspension, and achieve the effect of reducing harmful emissions, increasing fuel oxidation efficiency, and reducing harmful emissions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
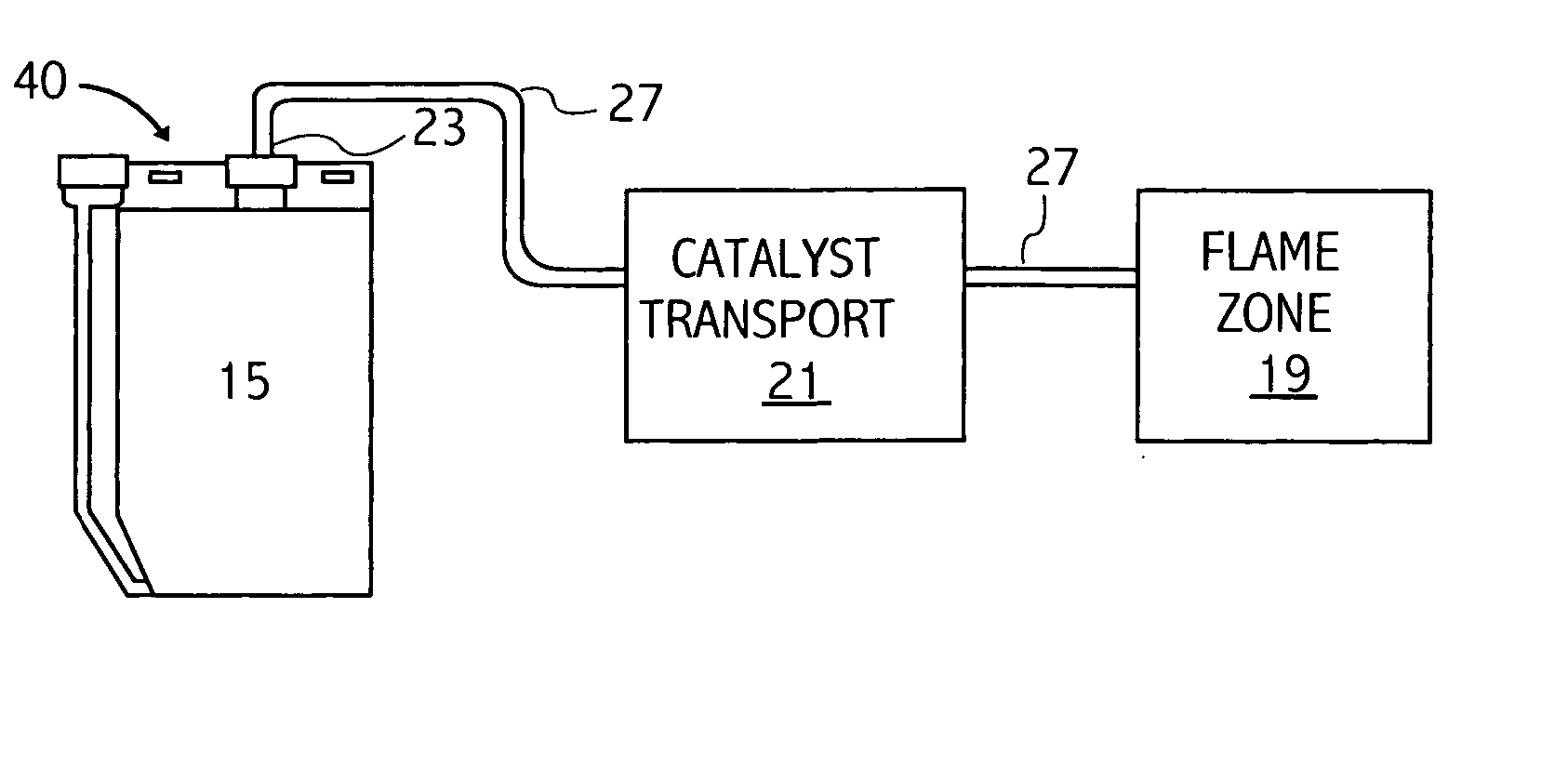
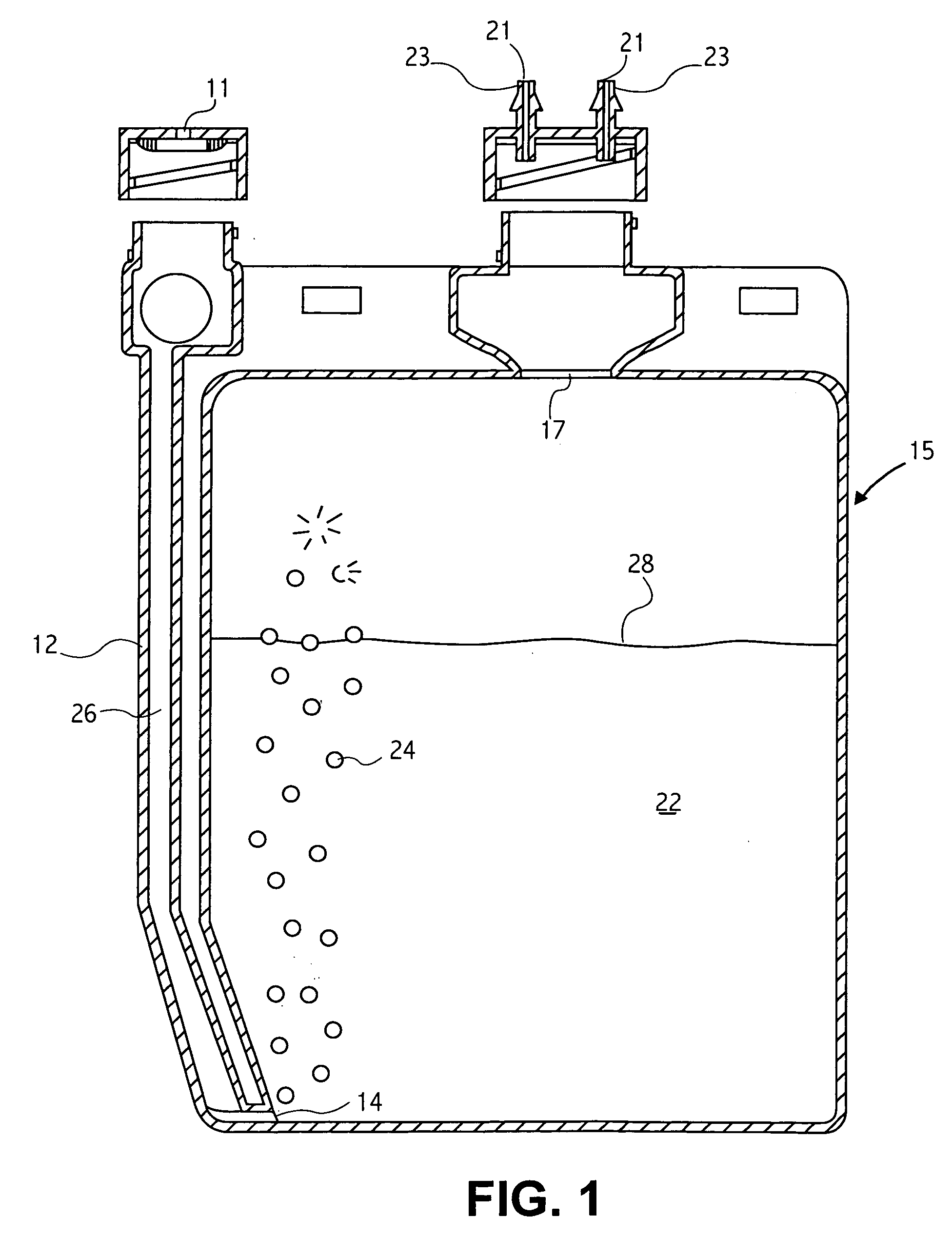
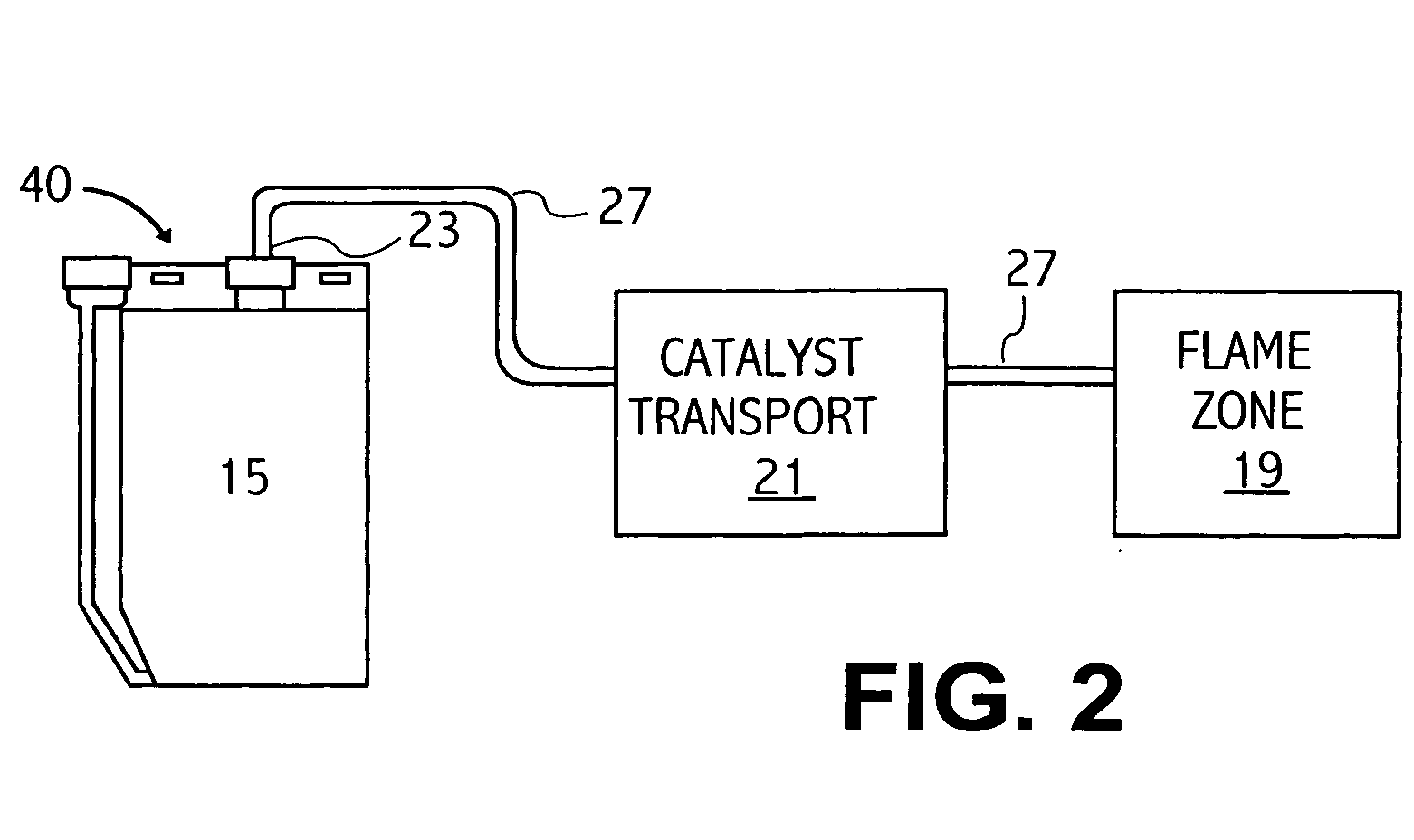
Referring to FIG. 3, a gasoline fuel may be oxidized in accordance with the method and composition disclosed herein. The current Example relates to a gasoline fuel engine having a fuel consumption rate of 5 gallons per hour operating for at least 400 hours, thereby consuming approximately two thousand (2,000) gallons (6056 kilograms) of fuel. A typical car consumes approximately 5 gallons / hour. The engine is provided with a catalyst delivery system as shown in FIG. 1. The specific catalyst mixture for a fuel rate of 5 gal / hour for this Example is as follows:
To one (1) liter of water was added:
2.4 g (2.4 milligrams / milliliter, mg / ml) H2PtCl6.6H2O (chloroplatinic acid) 0.4 g (0.4 mg / ml) HReO4 (perrhenic acid) 0.28 g (0.28 mg / ml) RhCl3 0.28 g (0.28 mg / ml) AlCl3.
The catalyst chamber 15 of the delivery system is charged with thirty six (36) ml of this catalyst mixture, and with enough of a base liquid comprising equal parts ethylene glycol and water and 5400 parts per million (...
example 2
The following specific tests were performed on a single Caterpillar Model No. 3408 Series Diesel Engine using a combined catalyst mixture comprising 144 ml of a mixture of water, 2.4 mg / ml H2PtCl6.6H2O, 0.4 mg / ml HReO4, 0.28 mg / ml RhCl3, and 0.28 mg / ml AlCl3, and a liquid comprising equal parts ethylene glycol and water and 5400 ppm by weight LiCl to bring the total of the combined catalyst mixture to 650 ml in the catalyst chamber. Using the same sparging process described in Example 1, the catalyst particles were carried to the combustion chamber. Unless otherwise indicated, the engine was running under an 85% load. Under the description provided in Example 1, 144 ml of catalyst mixture is sufficient for about 20 gal / hour of fuel consumption. Under 85% load, the Caterpillar engine ordinarily consumes approximately 23 gal / hour. Thus, the catalyst mixture was run lean for these tests. The tests discussed are standardized industry tests performed on appropriate calibrated equipment ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com