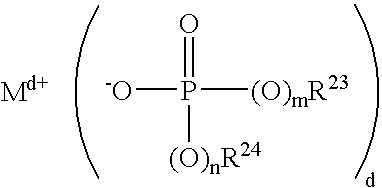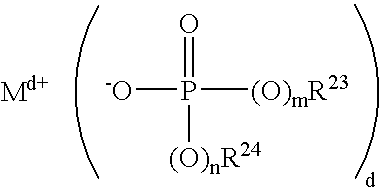Flame-retardant thermoset composition, method, and article
- Summary
- Abstract
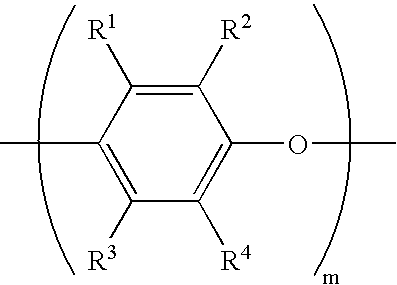
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
examples 1 and 2
, COMPARATIVE EXAMPLES 1-3
Several compositions were prepared and molded using the components and amounts summarized in Table 1, where all amounts are expressed as parts by weight (pbw). A methacrylate-capped poly(2,6-dimethyl-1,4-phenylene ether) resin (“Methacrylate-capped PPE”) having an intrinsic viscosity of about 0.3 deciliters per gram was prepared according to procedures described in U.S. Patent Application Publication No. 2001 / 0053820 A1 to Yeager et al. Fused silicas were obtained from Denka as FB-74 having an average particle size of 30.4 micrometers and a surface area of 1.6 meter2 / gram, and FS-20 having an average particle size of 5.0 micrometers and a surface area of 6.7 meter2 / gram. Hexanediol diacrylate was obtained from Sartomer as SR238. Trimethylolpropane trimethacrylate (TMPTMA) was obtained from Sartomer as SR350. Methacryloxypropyl trimethoxysilane (MAPTMS) was obtained from Dow Corning as Z-6030. A conductive carbon black was obtained as Printex XE-2 from Degu...
examples 3-11
A composition was prepared and molded according to the procedure described above. Cyclohexanedimethanol diacrylate was obtained from Sartomer as CD 406. A partially calcium saponified glycolic ester of montanic acid (montan wax) in a micronized form was obtained as CERIDUST® 5551 from Clariant. A fused silica having a median particle size of 17.7 micrometers and a surface area of 3.1 meter2 / gram was obtained as FB-570 from Denka. Another fused silica having a median particle size of 0.7 micrometers and a surface area of 6.2 meter2 / gram was obtained as SFP-30M from Denka. A colorant blend consisted of 57 parts of red colorant obtained as SANDOPLAST® Red G and 43 parts of green colorant obtained as SANDOPLAST® Green GSB, both from Clariant. Compositions are summarized in Table 2. Spiral flow length was determined using a spiral flow mold with a channel depth of 0.762 millimeters and a Gluco molding machine. Conditions used for the measurements were: platen temperature, 165° C.; mold ...
examples 12-17
, COMPARATIVE EXAMPLES 4-6
Seven compositions varying primarily in flame retardant type and amount were compounded, molded, and tested for flammability according to Underwriter's Laboratory procedure UL94. All samples contained silica, a colorant (carbon black or Keystone Green dye), a mold release agent (LICOWAX® S or LICOWAX® OP from Clariant), a flame retardant (melamine polyphosphate obtained from Ciba as MELAPUR® 200, aluminum tris(diethyl phosphinate) obtained from Clariant as OP930, or Clariant OP1311, which is believed to be a 9:1 weight / weight mixture of aluminum tris(diethyl phosphinate) and melamine polyphosphate), an initiator (dicumyl peroxide or t-butylperoxy benzoate), t-butylcatechol inhibitor, an acryloyl monomer (ethoxylated bisphenol A dimethacrylate obtained from Sartomer as SR348; 4-biphenyl methacrylate, CAS Reg. No. 46904-74-9, obtained from Hampford Research; or 4,4′-biphenol dimethacrylate, CAS Reg. No. 13082-48-9, prepared according to Liu et al., Polymer P...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com