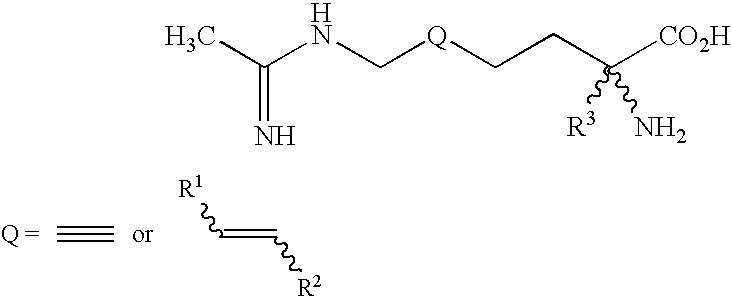
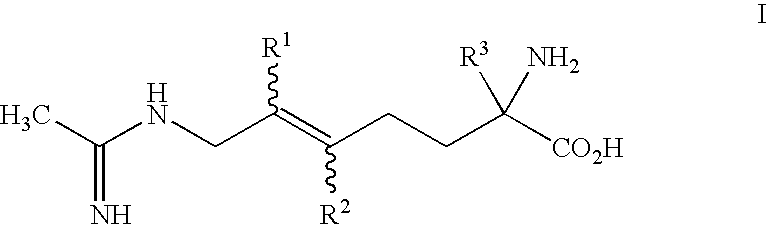
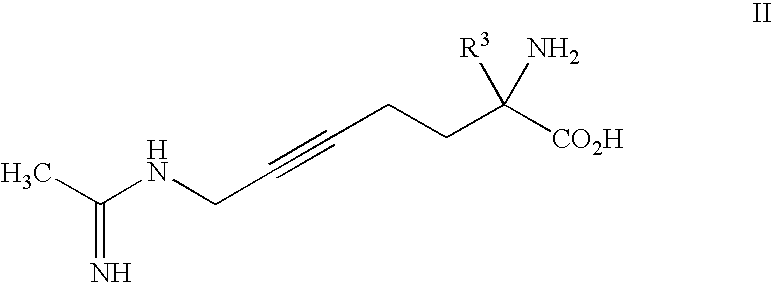
2-amino-2-alkyl-5 heptenoic and heptynoic acid derivatives useful as nitric oxide synthase inhibitors
a technology of nitric oxide synthase inhibitor and derivative, which is applied in the direction of drug composition, immunological disorders, metabolism disorders, etc., can solve the problem of unfavorable conformational rigidity with one or more carbon-carbon double bonds, and achieve the effect of reducing the risk or severity of opioid side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0470]
(2S,5E)-2-amino-2-methyl-6-fluoro-7-[(1-iminoethyl)amino]-5-heptenoic acid, dihydrochloride
[0471]
[0472] Example-1A) To a cold (−78° C.) solution of triethyl 2-fluorophosphonoacetate (25.4 g, 105 mmol) in 100 mL of THF was added n-butyl lithium (63 mL of 1.6 M in hexane, 101 mmol). This mixture was stirred at −78° C. for 20 min producing a bright yellow solution. A solution of crude 3-[(tert-butyldimethylsilyl)oxy]propanal (J. Org. Chem., 1994, 59, 1139-1148) (20.0 g, 105 mmol) in 120 mL of THF was then added dropwise over ten minutes, and the resulting mixture was stirred for 1.5 h at −78° C., at which time analysis by thin layer chromatography (5% ethyl acetate in hexane) showed that no starting material remained. The reaction was quenched at −78° C. with sat. aqueous NH4Cl (150 mL). The organic layer was collected, and the aqueous layer was extracted with diethyl ether (300 mL). The combined organics were washed with brine (200 mL), dried over MgSO4, filtered and concentrat...
example 2
[0499]
(2S,5E)-2-amino-2-methyl-6-fluoro-7-[(1-iminoethyl)amino]-5-heptenoic acid, dihydrochloride
[0500]
[0501] Example-2A) To a 1-methyl-2-pyrrolidinone (7500 mL) solution of methyl N-[(3,4-dichlorophenyl)-methylene]-alaninate (748.5 g, 2.88 mol) under nitrogen was added LiI (385.5 g, 2.88 mol) and the resulting slurry stirred approximately 20 minutes to give a clear solution. The solid from Example-1E (750 g, 2.40 mol) was then added and the resulting solution cooled in an ice bath to ˜0° C. Neat BTPP (900 g, 2.88 mol) was added dropwise over 25 minutes maintaining the internal temperature below 5° C. After stirring for an additional 1.5 hour at 5° C., the reaction was determined to be complete by HPLC. At this time, 7500 mL of methyl t-butyl ether (MTBE) was added followed by addition of 9750 mL of a water / crushed ice mixture. The temperature rose to 20° C. during this operation. After stirring vigorously for 5-10 minutes, the layers were separated and the aqueous layer washed wit...
example 3
[0522]
(2R,5E)-2-amino-2-methyl-6-fluoro-7-[(1-iminoethyl)amino]-5-heptenoic acid, dihydrochloride
[0523]
[0524] Example-3A) Separation of the individual enantiomers of the product from Example-2A was accomplished on preparative scale using chiral HPLC chromatography to give the desired pure (2R)-2-methyl amino ester product.
[0525] Example-3B) The product from Example-3A is dissolved in water and acetic acid. Zinc dust is added, and the mixture is heated at 60° C. until HPLC analysis shows that little of the starting material remains. The Zn is filtered through celite from the reaction mixture, and the filtrate is concentrated. The crude material is purified by reverse-phase HPLC column chromatography. Fractions containing product are combined and concentrated affording the desired (2R)-2-methyl acetamidine product.
[0526] Example-3) A solution of Example-3B in 2.0 N HCl is refluxed for 2 h. The solvent is removed in vacuo. The resulting solid is dissolved in water and concentrated ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com