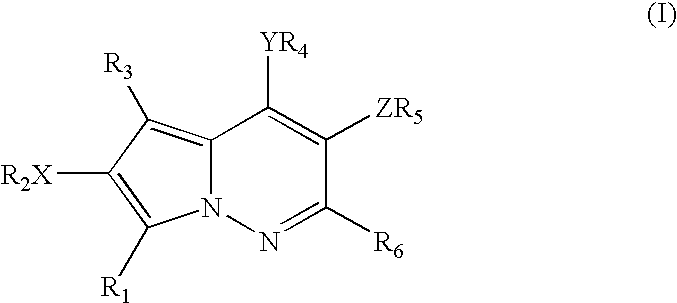
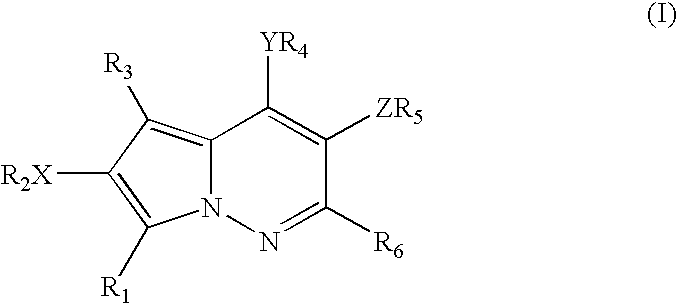
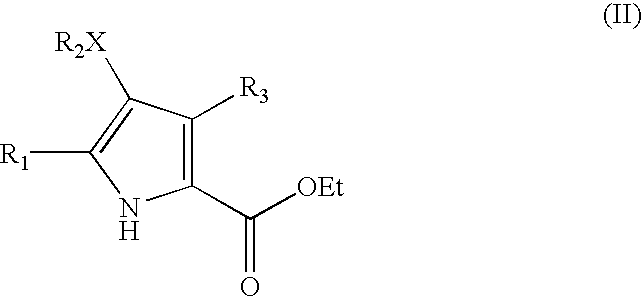
Pyrrolopyridazine compounds and methods of use thereof for the treatment of proliferative disorders
a technology of pyrrolopyridazine and compounds, which is applied in the direction of heterocyclic compound active ingredients, biocide, drug compositions, etc., can solve the problems of poor prognosis, high src activity, and loss of signal transduction normally exerted by the array of kinase enzymes in malignant cells,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of 3-Cyano-4-(cyclohexylamino)-5-methylpyrrolo[1,2-b]pyridazine-6-carboxylic acid ethyl ester (1E)
[0225]
A. Preparation of 3-Methyl-1H-pyrrole-2,4-dicarboxylic acid diethyl ester (1A)
[0226] To a solution of ethyl isocyanoacetate (38.1 mL, 0.34 mol) and DBU (50.8 mL, 0.34 mol) in THF (400 mL) at 50° C. was added a solution of acetaldehyde (9.5 mL, 0.17 mol) in THF (100 mL) over 25 min. The reaction mixture was stirred at 55° C. for 17 h, cooled to 25° C. and acetic acid (20 mL) was slowly added. The resulting mixture was concentrated in vacuo and the resulting residue was dissolved in ethyl acetate (800 mL) and washed with HCl (1 N, 3×300 mL). The combined aqueous washes were extracted with ethyl acetate (3×200 mL) and the combined organic layers were washed with NaHCO3 (sat. aq., 3×200 mL), water (100 mL) and brine (100 mL) and then concentrated in vacuo to afford a dark brown oil. Elution of this oil through a silica pad using ethyl acetate / hexanes (1:1) and the conc...
example 2
Preparation of 3-Cyano-5-methyl-4-phenoxypyrrolo [1,2-b]pyridazine-6-carboxylic acid ethyl ester
[0231]
[0232] To a solution of compound 1D (18 mg, 0.068 mmol) in acetonitrile (0.5 mL) at room temperature were added Et3N (21 uL, 0.205 mmol) and phenol (7 mg, 0.075 mmol). The reaction mixture was stirred for 24 h and then poured onto dichloromethane (10 mL) and NaHCO3 (sat. aq., 10 mL). The layers were separated, the aqueous phase was extracted with dichloromethane (3×5 mL), and the combined organic extracts were washed with water, dried over MgSO4, filtered, and concentrated in vacuo to afford compound 2 (15 mg, 68%) as a yellow solid. HPLC: 100% at 4.35 min (retention time) (YMC S5 ODS column, 4.6×50 mm, eluting with 10-90% aqueous methanol over 4 min containing 0.2% phosphoric acid, 4 mL / min, monitoring at 220 nm). MS (ES): m / z 340.0 [M+NH4]+.
example 3
Preparation of 6-(Methoxymethyl)-5-methyl-4-[(4-phenoxyphenyl)amino]pyrrolo[1,2-b]pyridazine-3-carbonitrile (3C)
[0233]
A. Preparation of 3-Cyano-5-methyl-4-[(4-phenoxyphenyl)amino]pyrrolo[1,2-b]pyridazine-6-carboxylic acid ethyl ester (3A)
[0234] To a solution of compound 1D (26 mg, 0.10 mmol) in DMF (2 mL) were added K2CO3 (138 mg, 1.00 mmol) and p-phenoxyaniline (20 mg, 0.11 mmol) at 25° C. The reaction mixture was stirred for 12 h and then diluted with dichloromethane (15 mL) and washed with water (10 mL) and brine (10 mL). The organic phase was dried over Na2SO4 and concentrated and the resulting residue was triturated with methanol to afford 31 mg (76% yield) of the desired compound as a yellow solid. HPLC: 100% at 4.62 min (retention time) (YMC S5 ODS column, 4.6×50 mm, eluting with 10-90% aqueous methanol over 4 min containing 0.2% phosphoric acid, 4 mL / min, monitoring at 220 nm). MS (ES): m / z 413.12 [M+H]+.
[0235] Compound 3A can also be prepared as follows: To a solution ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| retention time | aaaaa | aaaaa |
| retention time | aaaaa | aaaaa |
| retention time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com