Electrophoresis apparatus and method
a technology of electrophoresis and apparatus, applied in electrodialysis, refrigeration components, diaphragms, etc., can solve the problems of time-consuming and laborious separation of iefs, and achieve the effects of reducing the cost of operation, increasing separation speed, and scaling up capabilities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
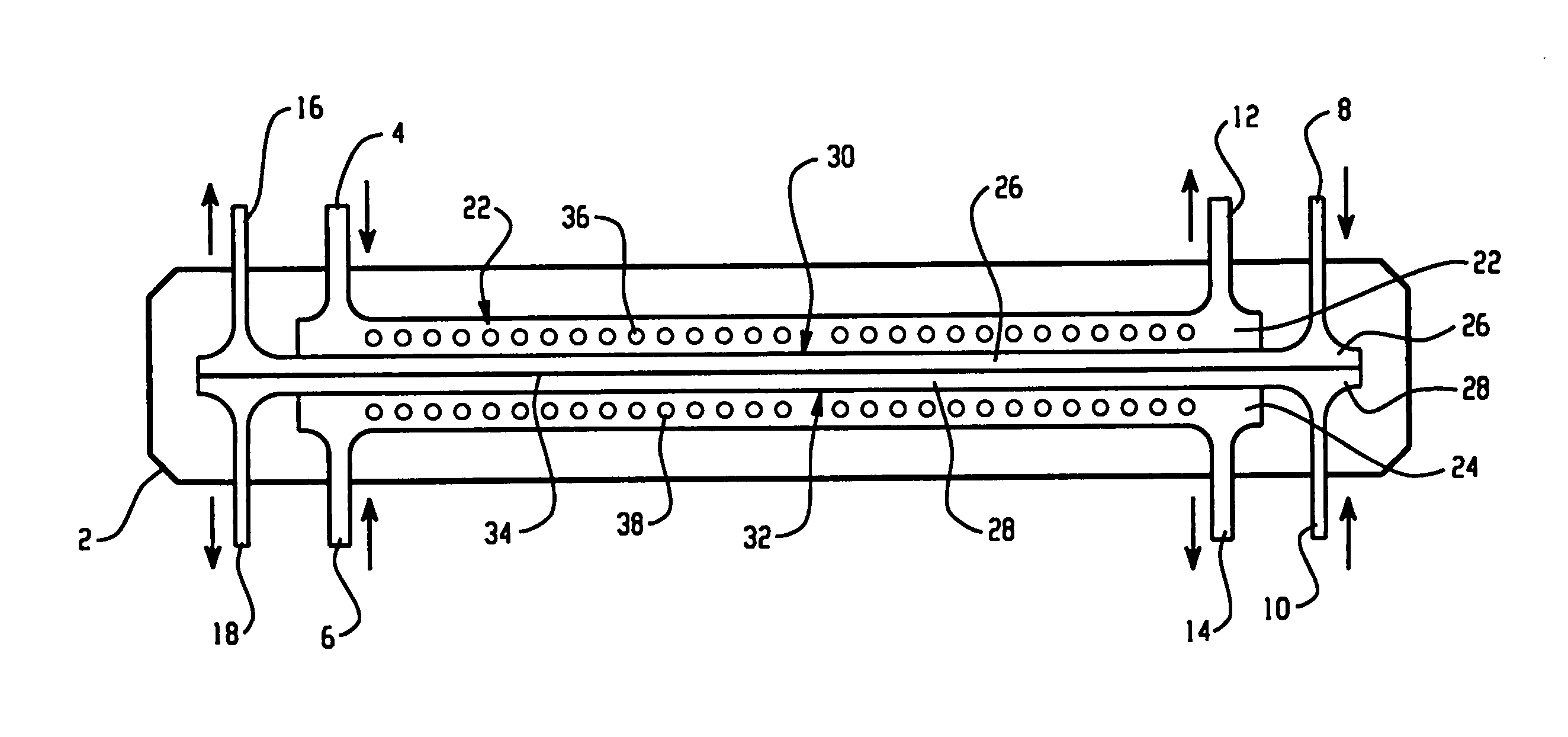
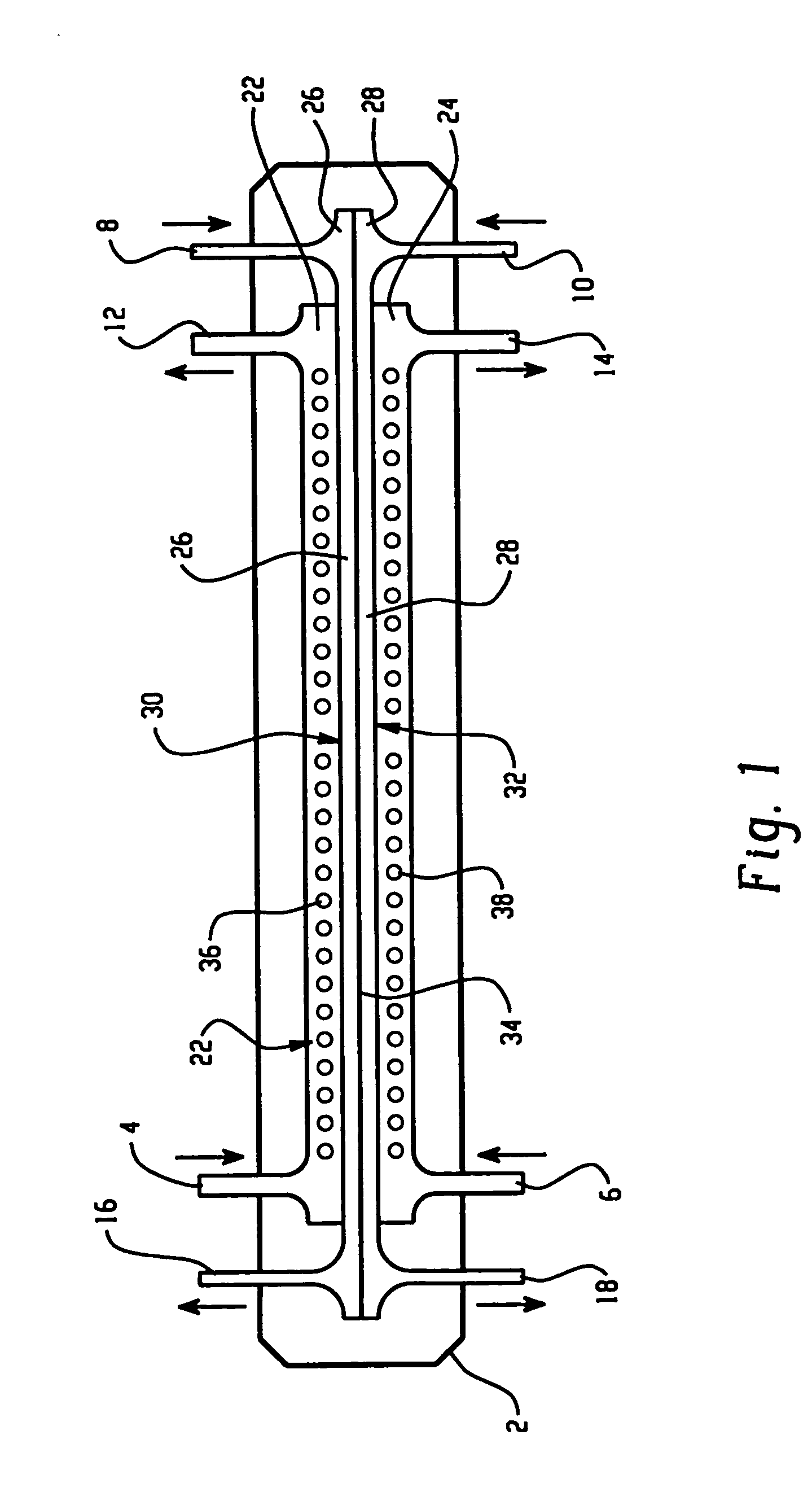
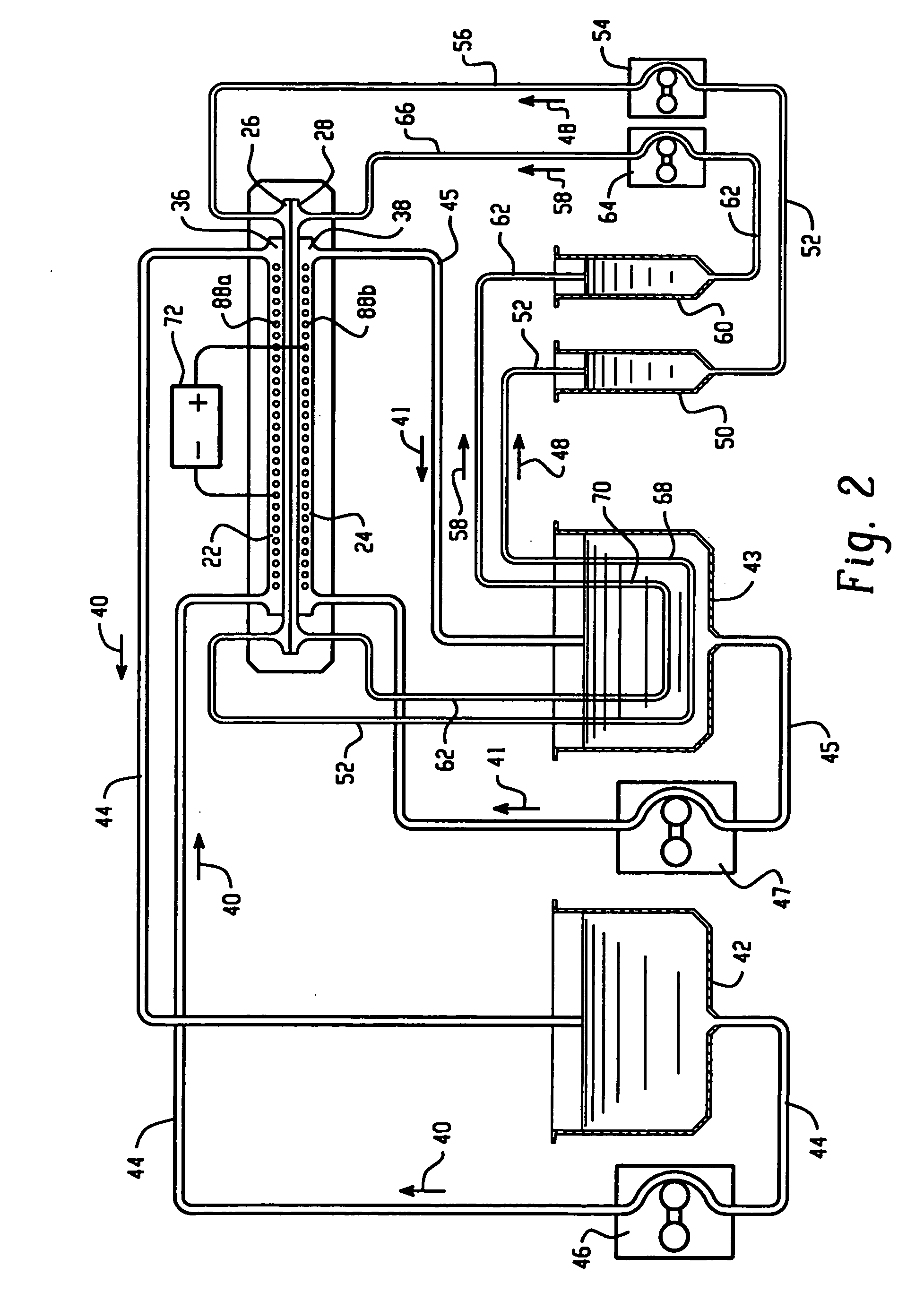
[0128] An apparatus according to the present invention, shown in FIG. 9, was used to separate the proteins present in chicken egg-white into two fractions. An electrophoresis separation cartridge, shown in FIGS. 3 to 7, was adapted to be used in the apparatus. The first ion-permeable barrier placed between the first electrolyte chamber and the first sample chamber was a pI=4.0 isoelectric membrane prepared from Immobiline chemicals (Pharmacia, Sweden), acrylamide and N-N′-methylene bis-acrylamide. The second ion-permeable barrier placed between the first sample chamber and the second sample chamber was a pI=5.0 isoelectric membrane prepared from Immobiline chemicals (Pharmacia, Sweden), acrylamide and N-N′-methylene bis-acrylamide. The third ion-permeable barrier placed between the second sample chamber and the second electrolyte chamber was a pI=7.0 isoelectric membrane prepared from Immobiline chemicals (Pharmacia, Sweden), acrylamide and N-N′-methylene bis-acrylamide.
[0129] The ...
example 2
[0133] The same apparatus as in Example 1 was used to separate the proteins present in chicken egg-white into two fractions. An electrophoresis separation cartridge, shown in FIGS. 3 to 7, was adapted to be used in the apparatus. The first ion-permeable barrier placed between the first electrolyte chamber and the first sample chamber was a polyacrylamide membrane with a nominal molecular mass cut-off of 5,000 dalton. The ion-permeable barrier between the first sample chamber and the second sample chamber was a pI 5.0 isoelectric membrane prepared from Immobiline chemicals (Pharmacia, Sweden), acrylamide and N-N′-methylene bis-acrylamide as in Example 1. The third ion-permeable barrier placed between the second sample chamber and the second electrolyte chamber was a polyacrylamide membrane with a nominal molecular mass cut-off of 5,000 dalton.
[0134] The first electrolyte reservoir was filled with 60 mL of a 2 mM acetic acid solution, pH 3.8. The second electrolyte reservoir was fill...
example 3
[0137] The same apparatus as in Example 1 was used to separate the proteins present in chicken egg-white into two fractions. The first ion-permeable barrier placed between the first electrolyte chamber and the first sample chamber was a polyacrylamide membrane with a nominal molecular mass cut-off of 1,000,000 dalton. The ion-permeable barrier between the first sample chamber and the second sample chamber was a pI 5.0 isoelectric membrane prepared from Immobiline chemicals (Pharmacia, Sweden), acrylamide and N-N′-methylene bis-acrylamide as in Example 1. The third ion-permeable barrier placed between the second sample chamber and the second electrolyte chamber was a polyacrylamide membrane with a nominal molecular mass cut-off of 1,000,000 dalton.
[0138] The first electrolyte reservoir was filled with 60 mL of a 2 mM acetic acid solution, pH 3.8. The second electrolyte reservoir was filled with 60 mL of an 8 mM triethanol amine solution, pH 9.9. The first and second sample reservoir...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular mass | aaaaa | aaaaa |
| molecular mass cut-off | aaaaa | aaaaa |
| molecular mass cut-off | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com