Treatment for insulin dependent diabetes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
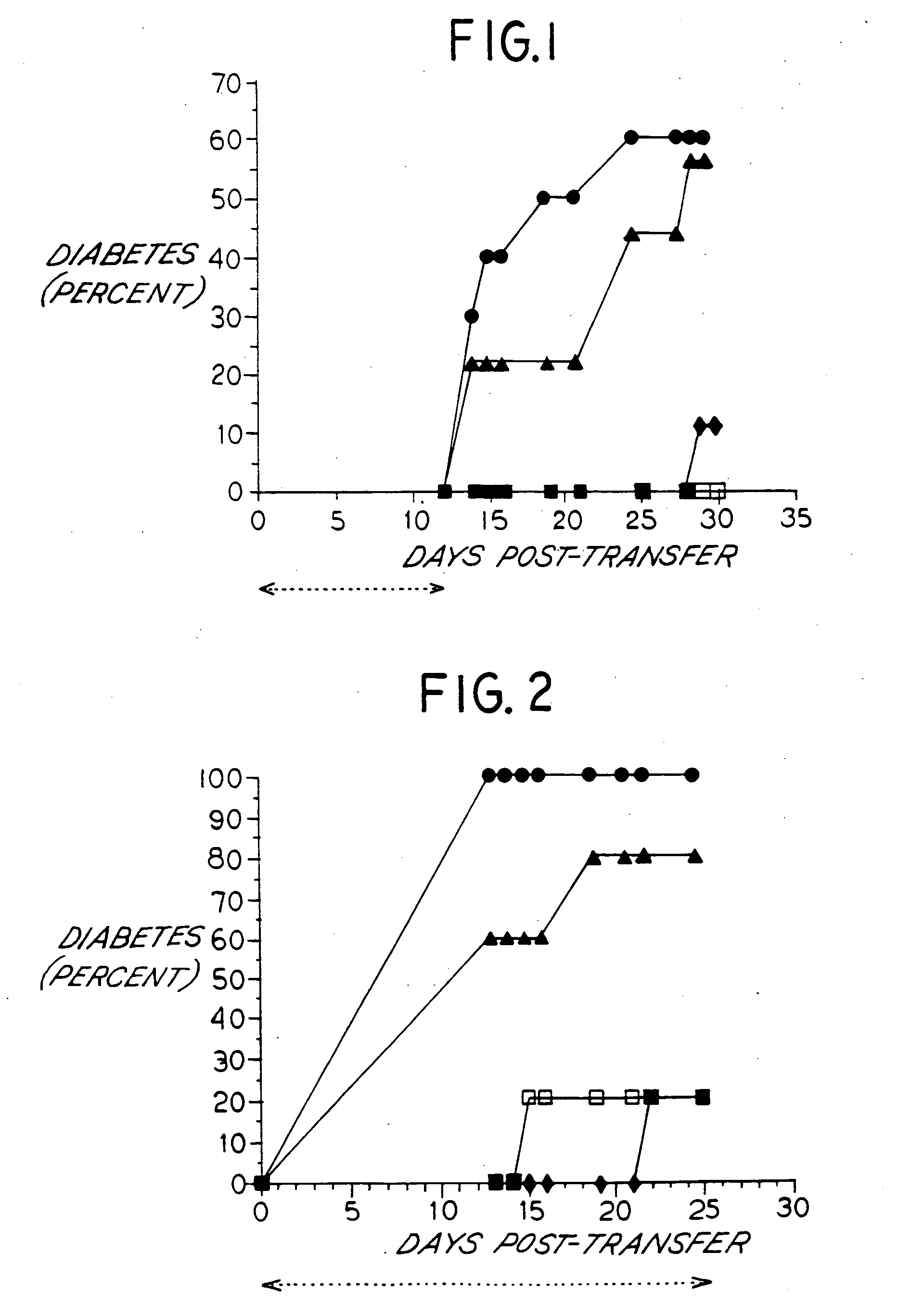
Effect of Anti-VLA-4 Antibody Treatment on Adoptive Transfer of Diabetes
[0048] For the adoptive transfer of diabetes experiments, NOD mice were obtained from Taconic Farms (Germantown, N.Y.) or from the Joslin Diabetes Center (Boston, Mass.). Spontaneously diabetic (D) females of recent onset (13-20 weeks of age) were used as spleen cell donors and 8 week old nondiabetic (Y) females served as recipients. Spleen cells from 4 week old nondiabetic (Y) female donors which fail to transfer disease were used as a negative control.
[0049] Recipient mice were placed on acidified water (1:8400 dilution of concentrated HCl in water) one week prior to sublethal irradiation (775 rad) performed in split doses (300 rad, 300 rad, and 175 rad) on each of three days (day-2, -1, and the day of transfer), in order to minimize any incidence of intestinal infection subsequent to high dose irradiation (Gamma Cell 1000 Cesium 137 source, Nordion International, Inc., Ontario, Canada). Spleens were harvest...
example 2
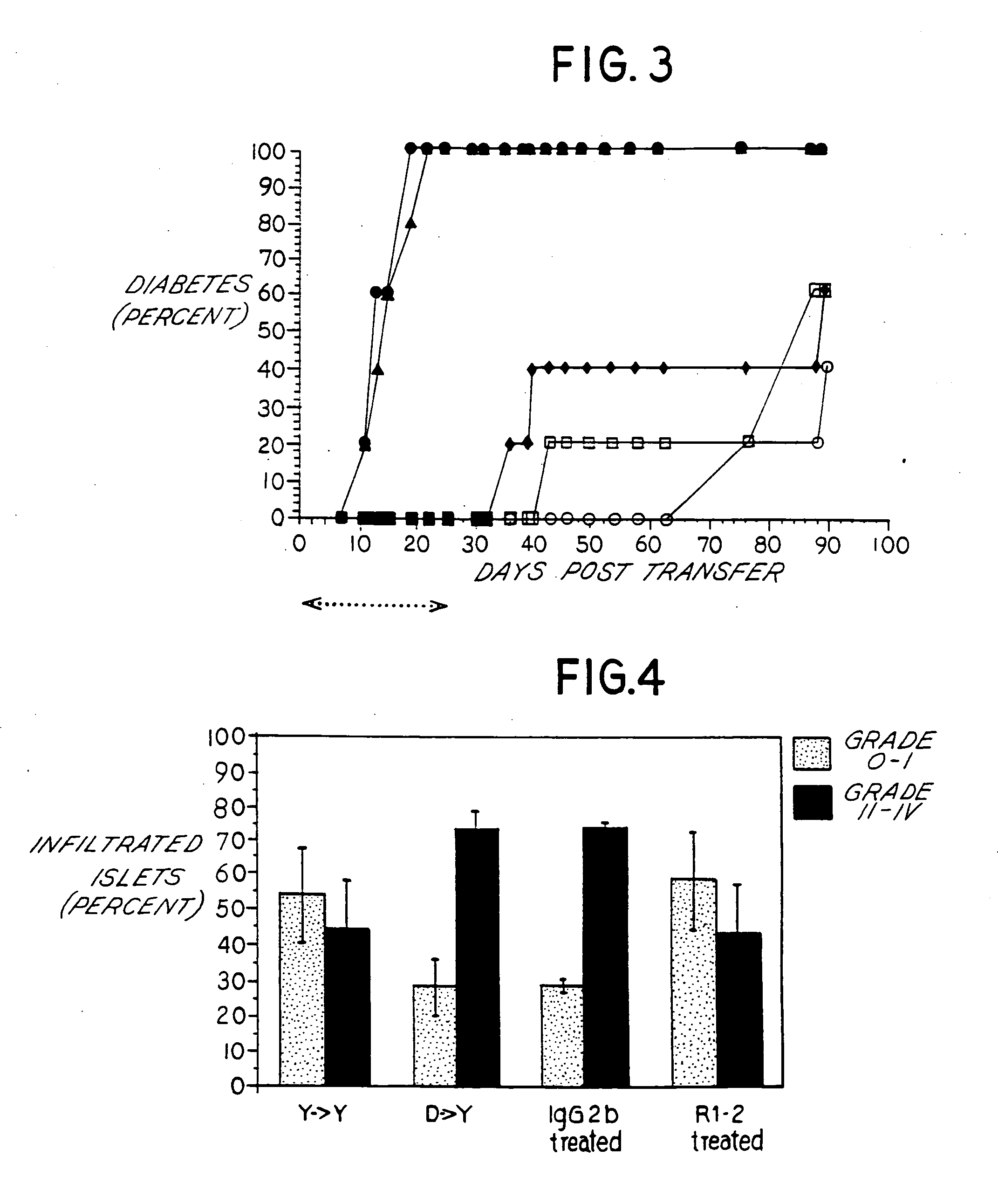
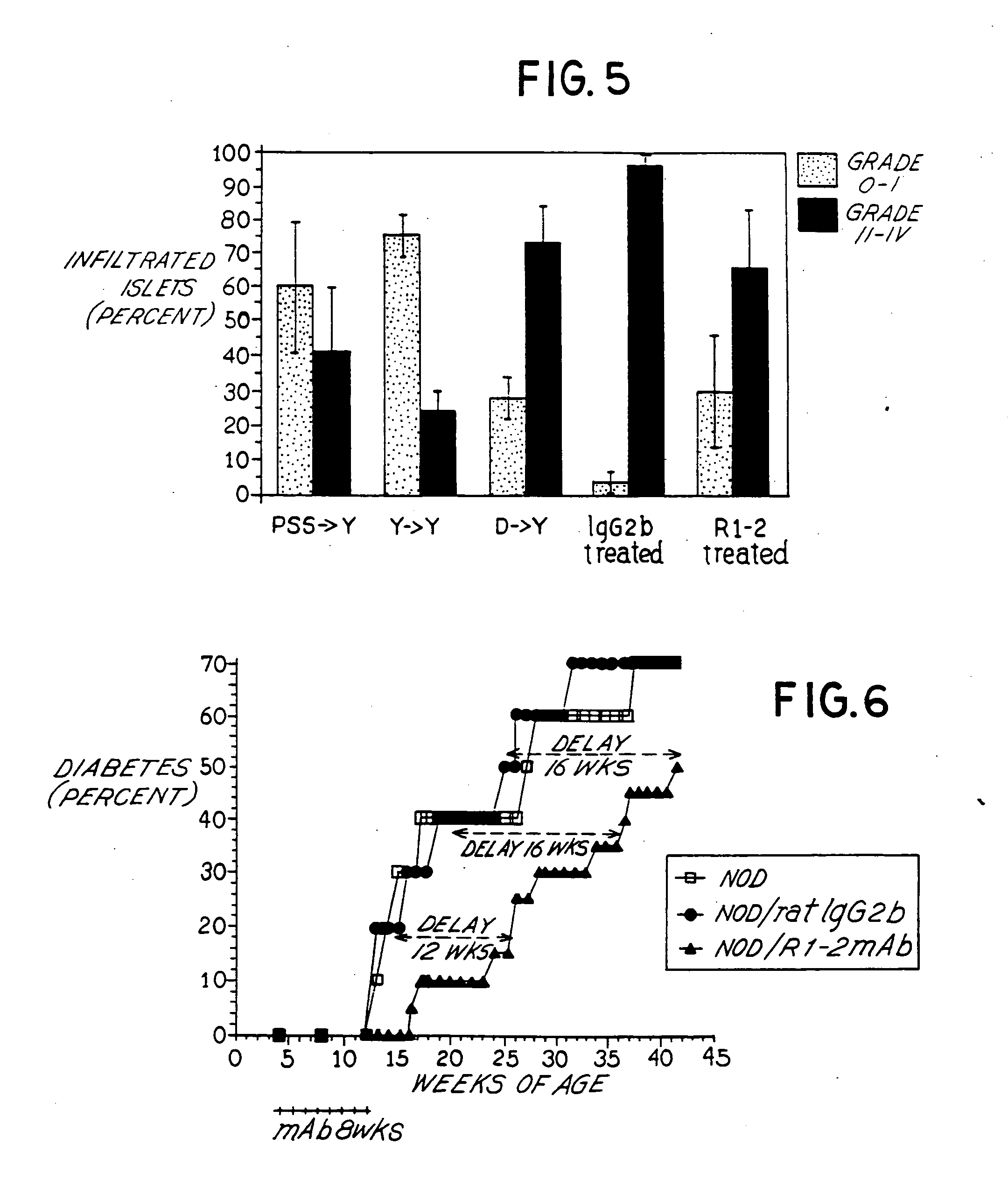
Effect of Anti-VLA-4 mAb on Pancreatic Insulitis
[0056] For histological analysis, mice were sacrificed between 2-4 weeks post-transfer as described in this Example and pancreata harvested in 10% formalin buffered saline for paraffin-embedded sections which were stained with hematoxylin and eosin (H&E) for histology. Degree of insulitis was scored as follows: Grade 0: no insulitis [islet devoid of inflammation]; Grade I: peri-insulitis [inflammatory mononuclear cells located peripheral to the islet]; Grade II: 50% infiltrated. The percent of islets in each Grade was then calculated relative to the total number of islets examined. Histologic sections were examined and scored for the degree of insulitis following the adoptive transfer of NOD splenocytes with and without anti-VLA-4 mAb treatment and the results tabulated. Specifically, the frequency of uninfiltrated islets (Grade 0-I infiltrate) and islets with Grade II-IV insulitis (as described above) were quantitated. For each exper...
example3
Comparison of Different Anti-VLA-4 Antibody Treatment on Adoptive Transfer of Diabetes
[0059] This Example provides comparative efficacy results of PS / 2, an anti-VLA-4 antibody, with R1-2 using the adoptive transfer model and procedure described in Example 1. NOD mice were treated with (a) an irrelevant control antibody (D / rat IgG2b, n=19 mice); (b) R1-2 antibody (D / R1-2 mAb, n=24 mice); (c) PS / 2 mAb (D / PS / 2 mAb, n=5 mice); or (d) no treatment (NONE, n=26 mice). Spleen cells were injected intravenously (2-3×107 in 0.2 ml PBS) and pretreated with either 75 μg R1-2 mAb, 75 μg PS / 2 mAb, 75 μg rat IgG2b, or untreated. Isolation and purification of PS / 2 anti-VLA-4 mAb was originally described by Miyake et al., 1991 [55].
[0060] The R1-2 mAb, PS / 2 mAb or rat IgG2b was administered at a dose of 75 μg / 0.2 ml intraperitoneally every 2-3 days, a dosing regimen which was determined to maintain maximal coating of VLA-4-positive cells in the peripheral blood, lymphoid organs and bone marrow as d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com