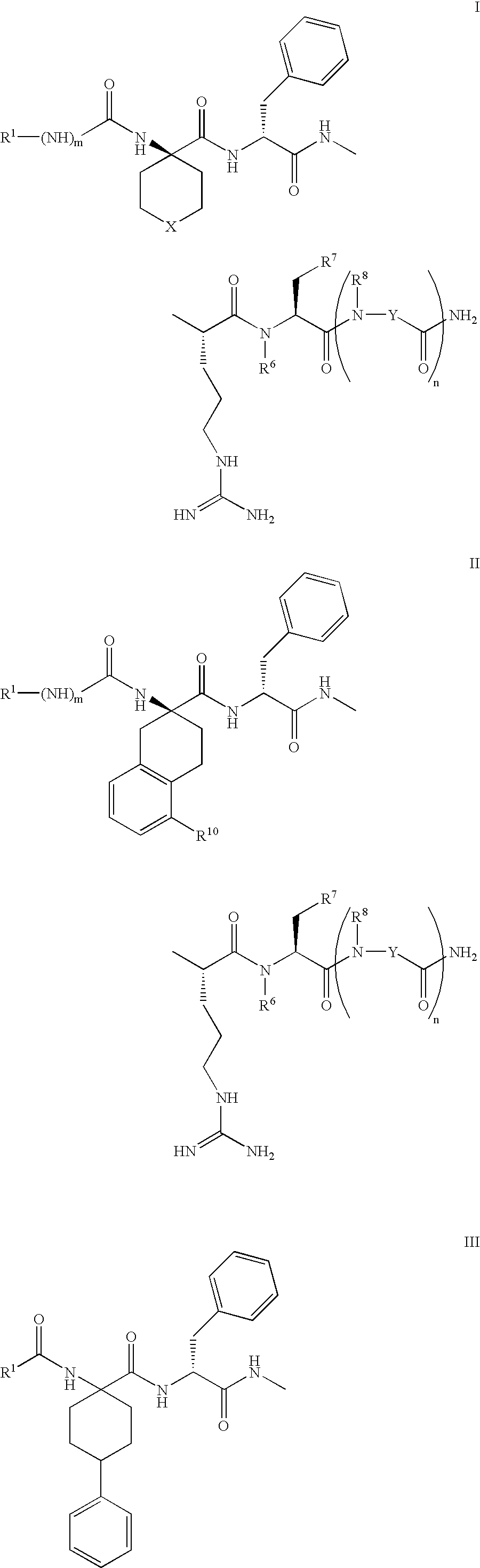
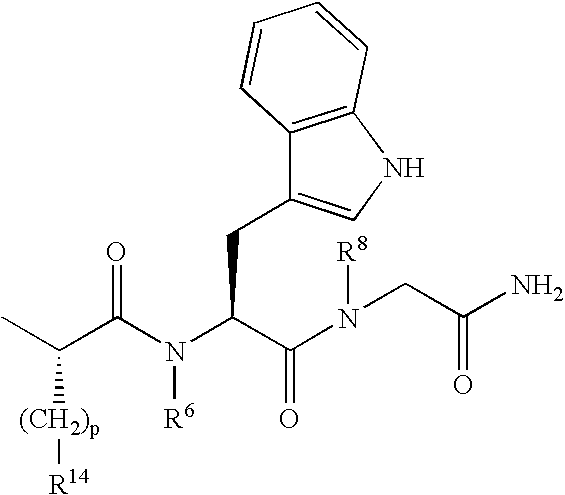
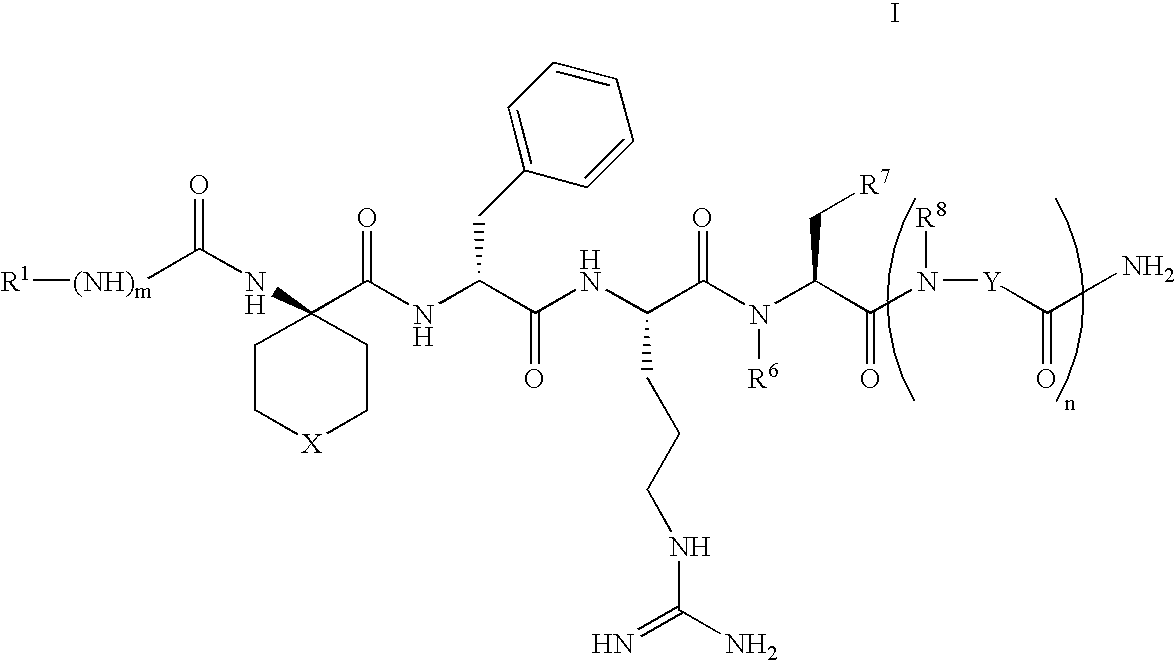
Selective linear peptides with melanocortin-4 receptor (MC4-R) agonist activity
a melanocortin-4 receptor and selective linear peptide technology, applied in the direction of peptide/protein ingredients, drug compositions, metabolic disorders, etc., can solve the problems of increasing the risk of skin cancer, increasing the risk of adrenal tissue carcinoma, and presenting a significant burden on the health care system, so as to improve the mc4-r agonist activity and the effect of treating or preventing obesity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Fmoc-1-amino-4-phenylcyclohexane-1-carboxylic acid (Fmoc-Apc-OH)
Step 1:
To a solution of 4-phenylcyclohexanone (10.0 g, 57.5 mmol) in ethanol (100 mL) and water (33 mL) in a glass pressure bottle, were added ammonium carbonate (33 g, 344 mmol, 6 equiv.) and potassium cyanide (5.6 g, 86.2 mmol, 1.5 equiv.). The mixture was heated at 80-90° C. for 24 hrs. The cooled reaction mixture was added to icy water (400 ml) and stirred vigorously for 30 min. The resulting precipitate was suction filtered, washed thoroughly with water and dried to yield the hydantoin as a white solid (14.0 g, 100% yield). 1H NMR (DMSO-d6): 8.63 (s, 1H), 7.23-7.36 (m, 4), 7.15 (m, 1), 2.50 (m, 1H), 2.10 (m, 1H), 1.85 (d, 1H) and 1.55-1.80 (m, 6H).
Step 2:
The hydantoin (10.0 g) was suspended in aqueous NaOH (6N, 350 mL) and heated at 130° C. for 2-3 days. Upon the completion of the hydrolysis, the reaction mixture was neutralized with conc. HCl to slightly acidic (pH ˜6). The resulting slurr...
example 2
Preparation of Fmoc-1-amino-4-(4-methoxyphenyl)cyclohexane-1-carboxylic acid (Fmoc-4-MeOApc-OH)
Step 1:
A solution of 4-(4-hydroxyphenyl)cyclohexanone (5.0 g, 26.3 mmol) in acetone (100 mL) was treated with K2CO3 (14.5 g, 105 mmol, 4 equiv) and iodomethane (4.9 mL, 11.2 g, 78.6 mmol, 3 equiv.). The reaction was heated at 65° C. overnight. After the solvent was removed, the residue was treated with H20 and extracted with EtOAc. The organic extracts were combined and washed with brine, dried over Na2SO4 and concentrated in vacuum to give the spectroscopically pure 4-(4-methoxyphenyl)cyclohexanone (5.34 g, 100%). 1HNMR(CDCl3) 7.16 (dt, 2H), 6.87 (dt, 2H), 3.78 (s, 3H), 2.99 (tt, 1H), 2.47-2.53 (m, 4H), 2.20 (m, 2H) and 1.83-1.98 (m, 2H); MS (electrospray) m / e, 205 (M+1)+, Calcd for C13H16O2, 204.
Step 2:
To a solution of the ketone (3.86 g, 18.9 mmol) in ethanol (50 mL) and water (15 mL) in a glass pressure bottle, were added ammonium carbonate (14.5 g, 151 mmol, 8 equiv.) and po...
example 3
Preparation of Fmoc-1-amino-4-(4-ethoxyphenyl)cyclohexane-1-carboxylic acid (Fmoc-4-EtOApc-OH)
Step 1:
A solution of 4-(4-hydroxyphenyl)cyclohexanone (5.0 g, 26.3 mmol) in acetone (100 mL) was treated with K2CO3 (14.5 g, 105 mmol, 4 equiv) and iodoethane (10.5 mL, 20.5 g, 131 mmol, 5 equiv.). The reaction was heated at 65° C. overnight. After the solvent was removed, the residue was treated with H2O and extracted with EtOAc. The organic extracts were combined and washed with brine, dried over Na2SO4 and concentrated in vacuum to give the spectroscopically pure 4-(4-ethoxyphenyl)cyclohexanone (5.74 g, 100%). 1H NMR (CDCl3) 7.15 (dt, 2H), 6.86 (dt, 2H), 4.02 (q, 2H), 2.99 (tt, 1H), 2.46-2.54 (m, 4H), 2.16-2.24 (m, 2H), 1.83-2.00 (m, 2H) and 1.41 (t, 3H); MS (electrospray) m / e, 219 (M+1)+, Calcd for C14H18O2, 218.
Step 2:
To a solution of the ketone (4.15 g, 19.01 mmol) in ethanol (50 mL) and water (15 mL) in a glass pressure bottle, were added ammonium carbonate (14.5 g, 151 mmo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com