Expression of functional antibody fragments
a functional antibody and fragment technology, applied in the field of functional antibody fragment production, can solve the problems of difficult control of the precise nature and proportion of the recovered antibody fragment, and the reaction involves highly toxic compounds, and achieve the effect of facilitating the preparation of homogeneous recombinan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
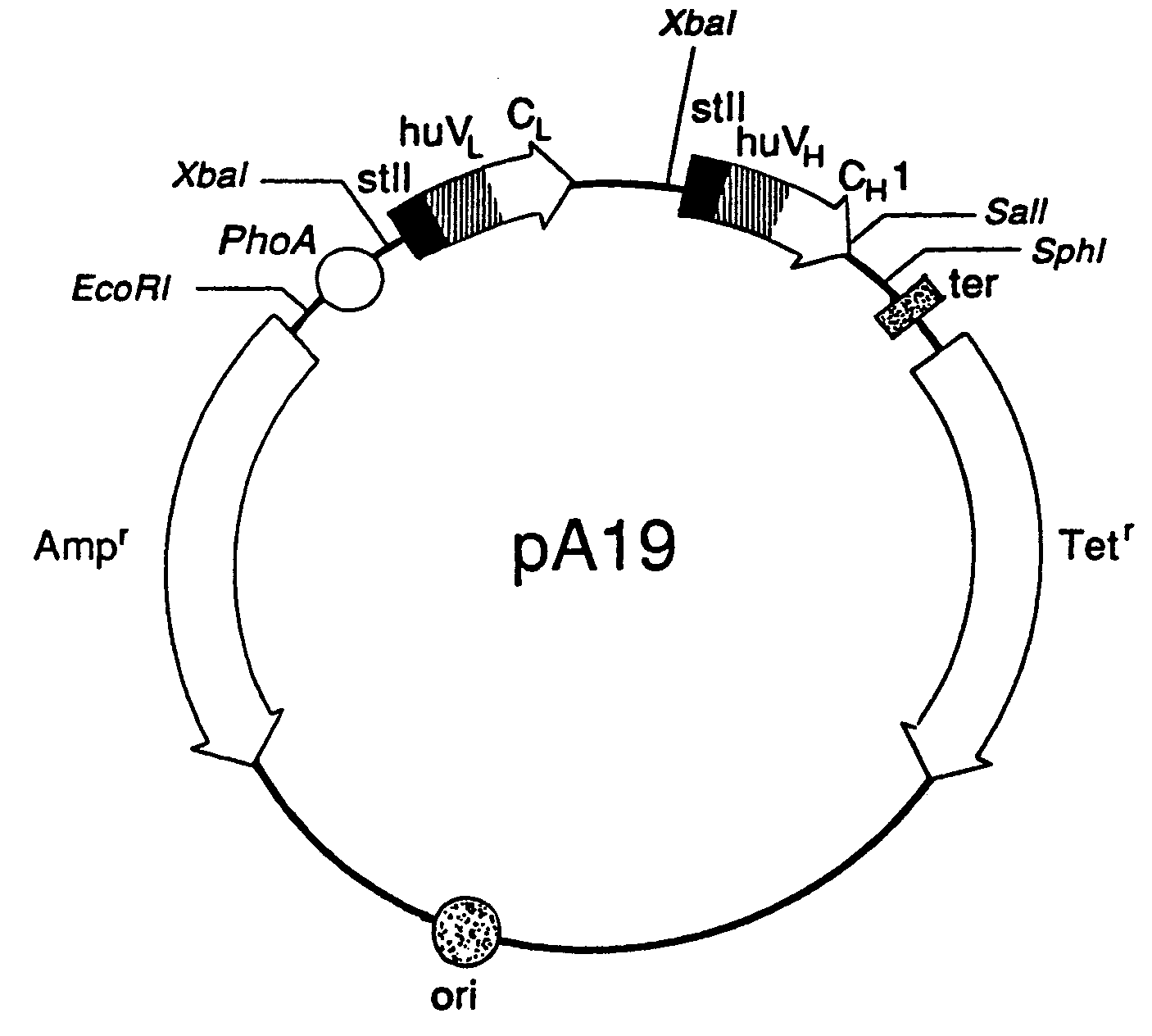
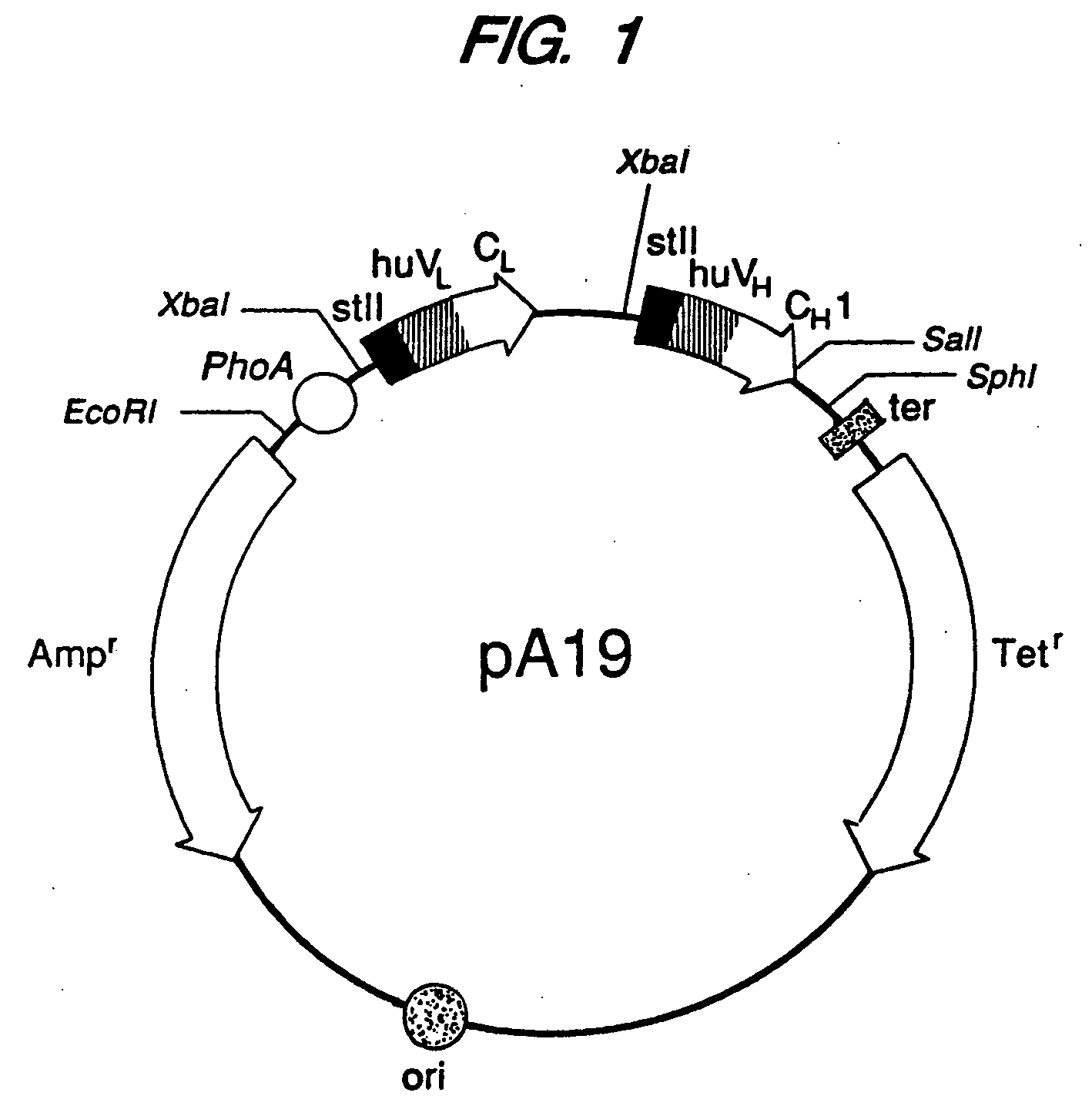
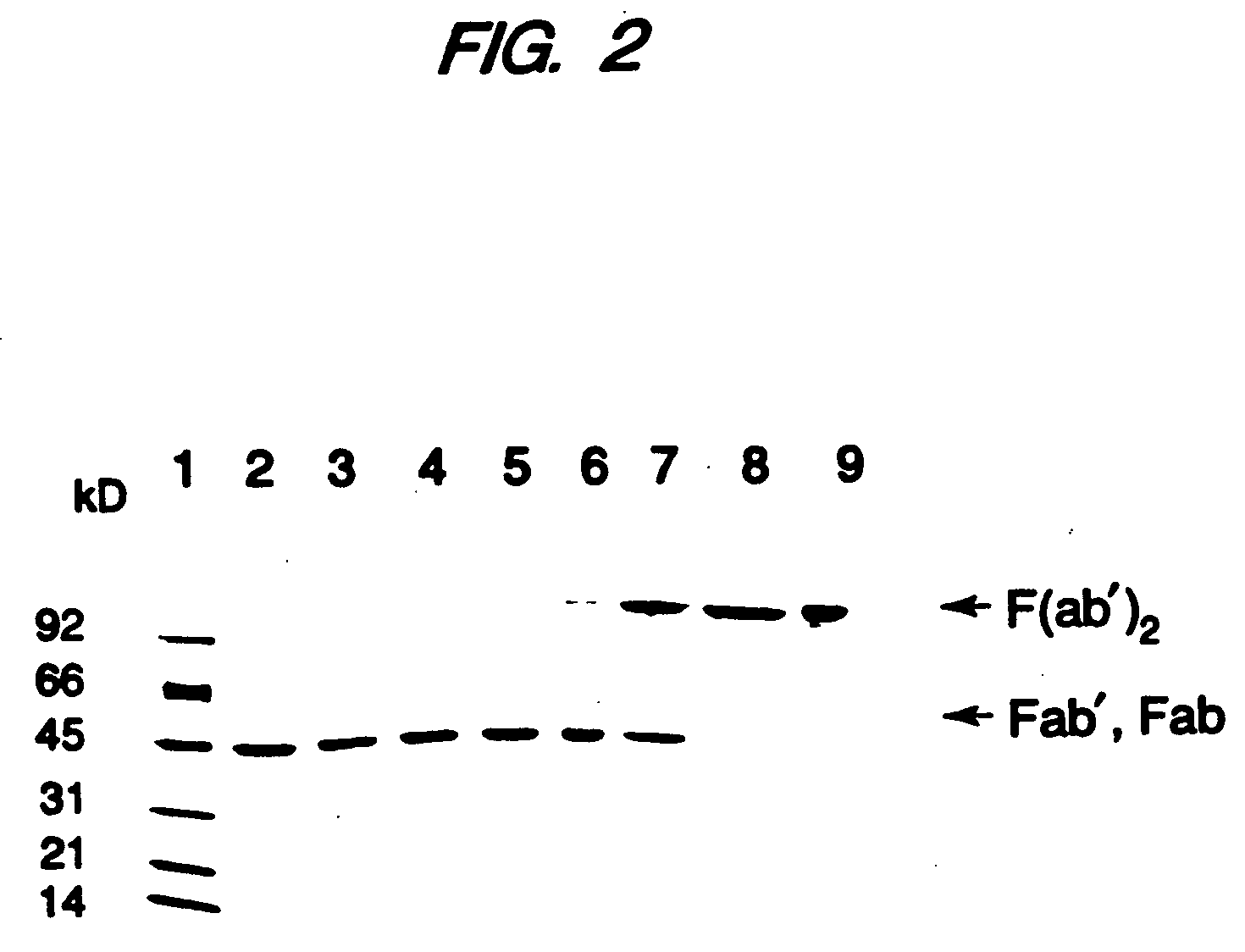
Expression of Active Fab, Fab′, and F(ab′)2, Antibody Fragments
[0115] Overexpression of the HER2 proto-oncogene product (p185HER2) has been associated with a variety of aggressive human malignancies. An Escherichia coli expression system has been developed that secretes functional Fab and Fab′ fragments of a humanized antibody, huMAb4D5-8, at titers of about 1 to in excess of about 2 grams per liter as judged by binding to antigen, p185HER2. The Fab′ fragment was recovered with the single hinge region cysteine present mainly as the free thiol (up to about 90 mole %) permitting efficient directed disulfide bond formation in vitro to form the bivalent F(ab′)2 antibody fragment. This molecule is indistinguishable from F(ab′)2 derived from proteolysis of intact antibody in antigen binding affinity and in anti-proliferative activity against the human breast tumor cell line, SK-BR-3, which over-expresses p185HER2, but unlike the proteolytic product, the F(ab′)2 here is C-terminally homog...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com