Patient oxygenation using stabilized fluorocarbon emulsions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0071] Preparation of Reference Emulsion
Composition of Reference Emulsion:
Perflubron / Lecithin (90 / 4% w / v)
[0072] A reference emulsion containing 90 g PFOB, 4 g egg yolk phospholipid (EYP), and physiological levels of salts and buffers was prepared by high pressure homogenization according to the method of Long (U.S. Pat. No. 4,987,154).
example 2
[0073] Stabilization of a 90% w / v Fluorocarbon Emulsion (Perfluorooctyl Bromide / Perfluorodecyl Bromide)
[0074] The protocol of Example 1 was repeated to form four additional emulsions, except that in successive emulsions, the fluorocarbon was perfluorooctyl bromide containing 1%, 2%, 5%, and 10% perfluorodecyl bromide (w / w), respectively.
example 3
[0075] Emulsion Stability
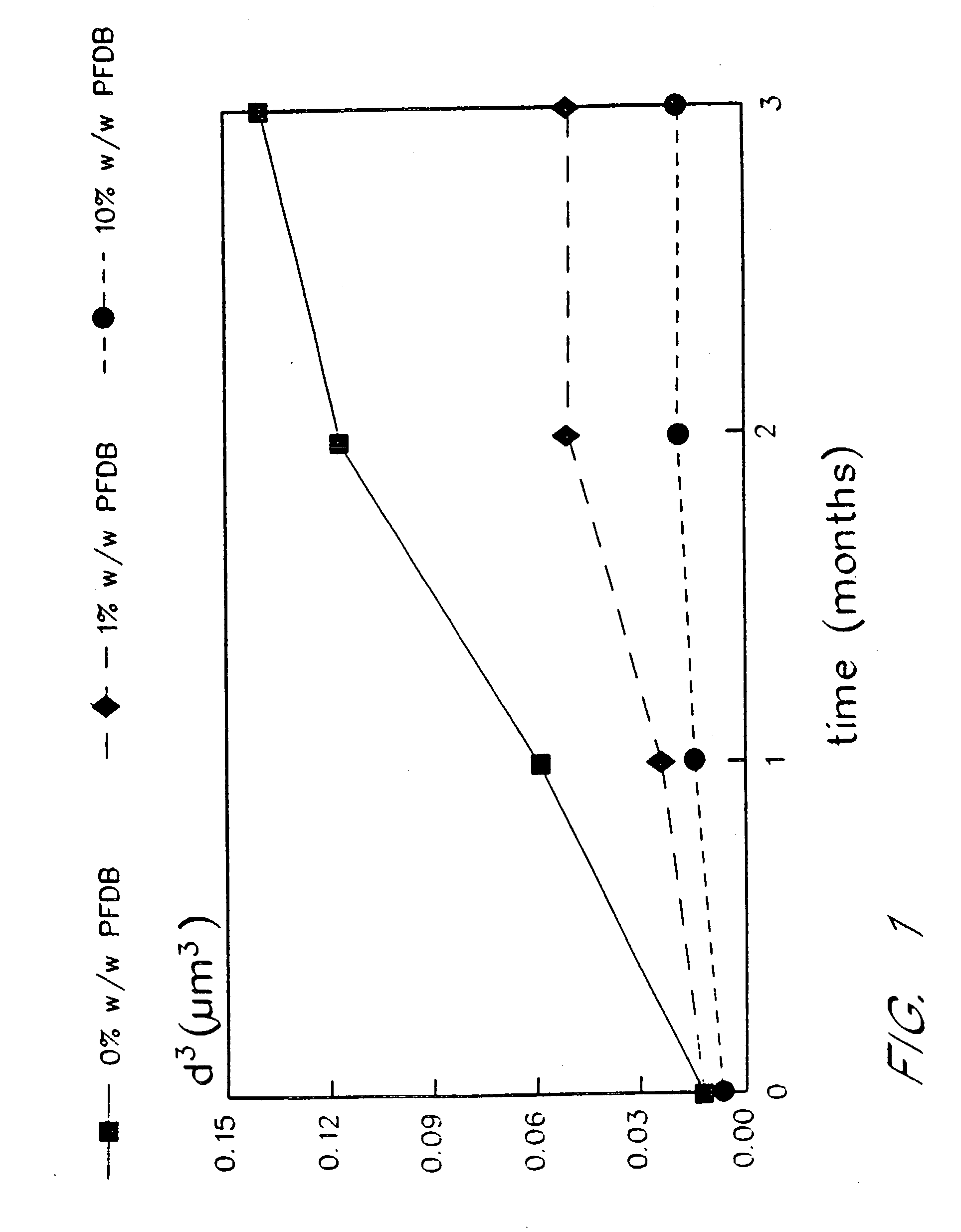
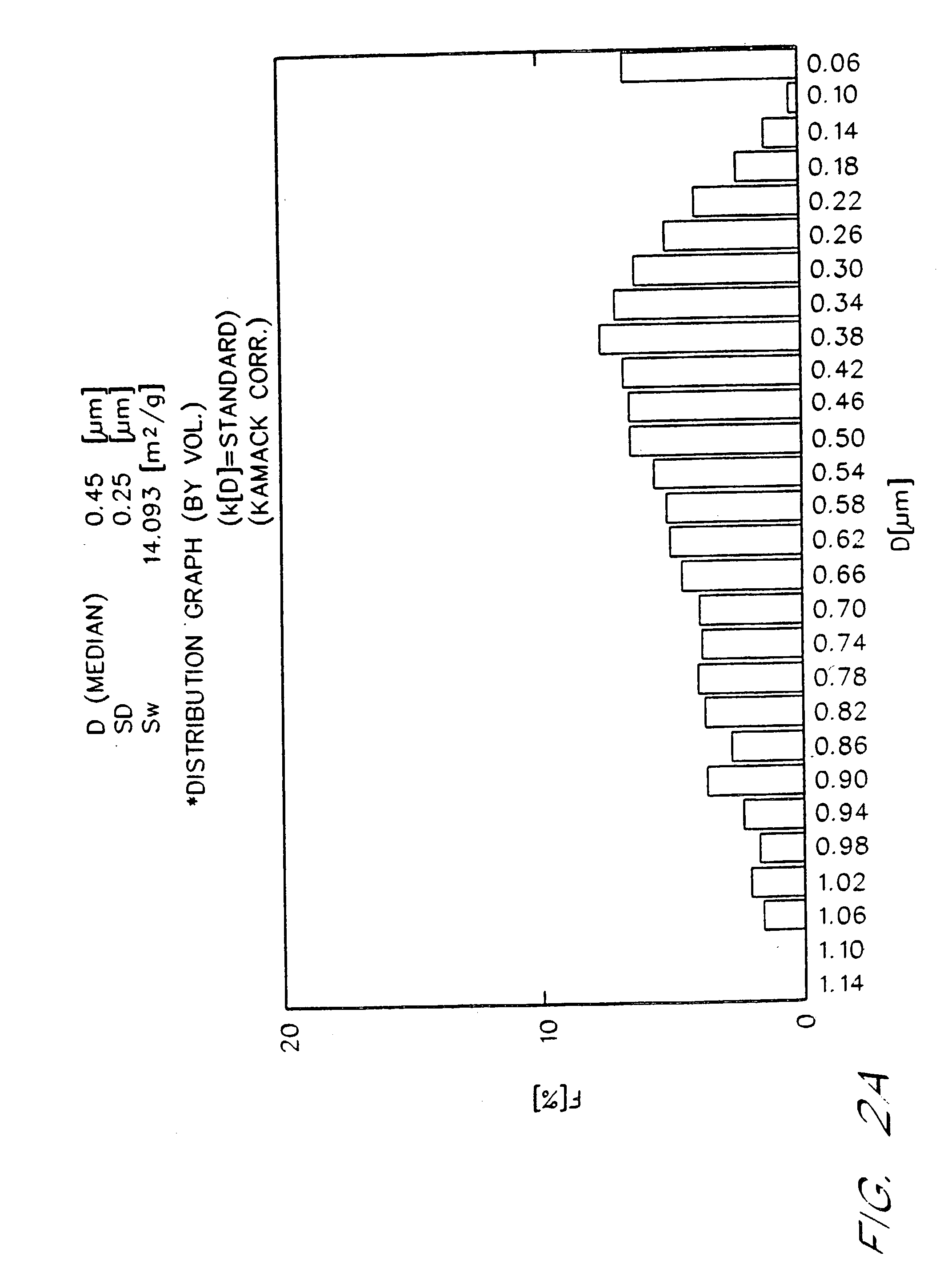
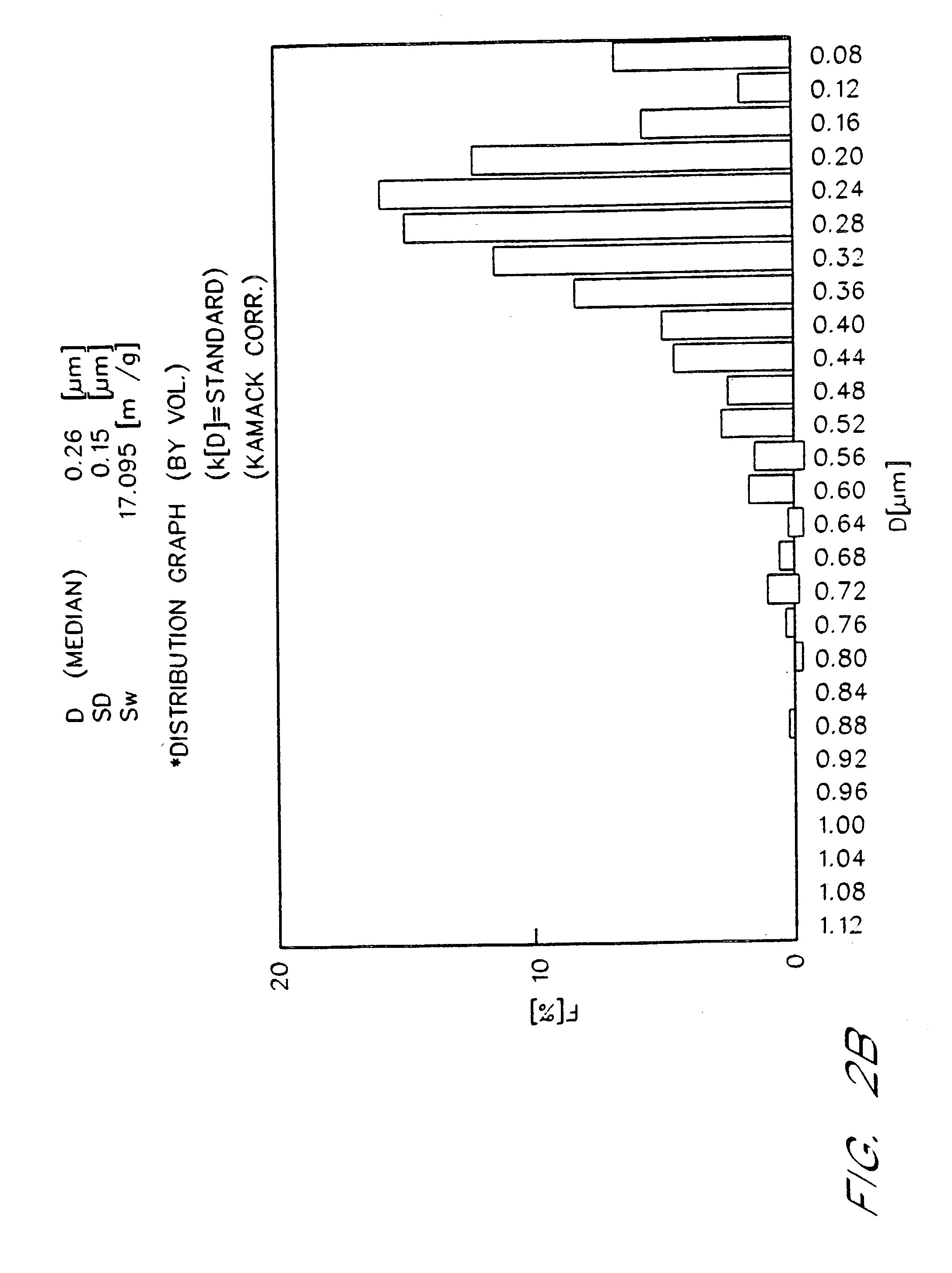
[0076] The emulsions prepared by the procedures of Examples 1 and 2 were placed on accelerated stability testing at 40EC for three months. Table I demonstrates particle size stability over time for 90% (w / v) fluorocarbon emulsions. Such emulsions include a control, in which 100% of the fluorocarbon phase is perfluorooctyl bromide, and emulsions of the present invention in which the fluorocarbon phase is 99% to 90% w / w perfluorooctyl bromide, with from 1% to 10% w / w of perfluorodecyl bromide added as a stabilizer. In FIG. 1 and Table I, “EYP” is egg yolk phospholipid, “perflubron” is perfluorooctyl bromide, “PFDB” is perfluorodecyl bromide, and “S” is the rate of particle growth in units of μm3 / mo. FIG. 1 illustrates typical Lifshitz-Slezov graphs of d3 as a function of time for these emulsions. The cubed term is chosen for the ordinate since Lifshits-Slezov theory predicts that plots of d3 vs time will yield a straight line. In fact, this linear dependence ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com