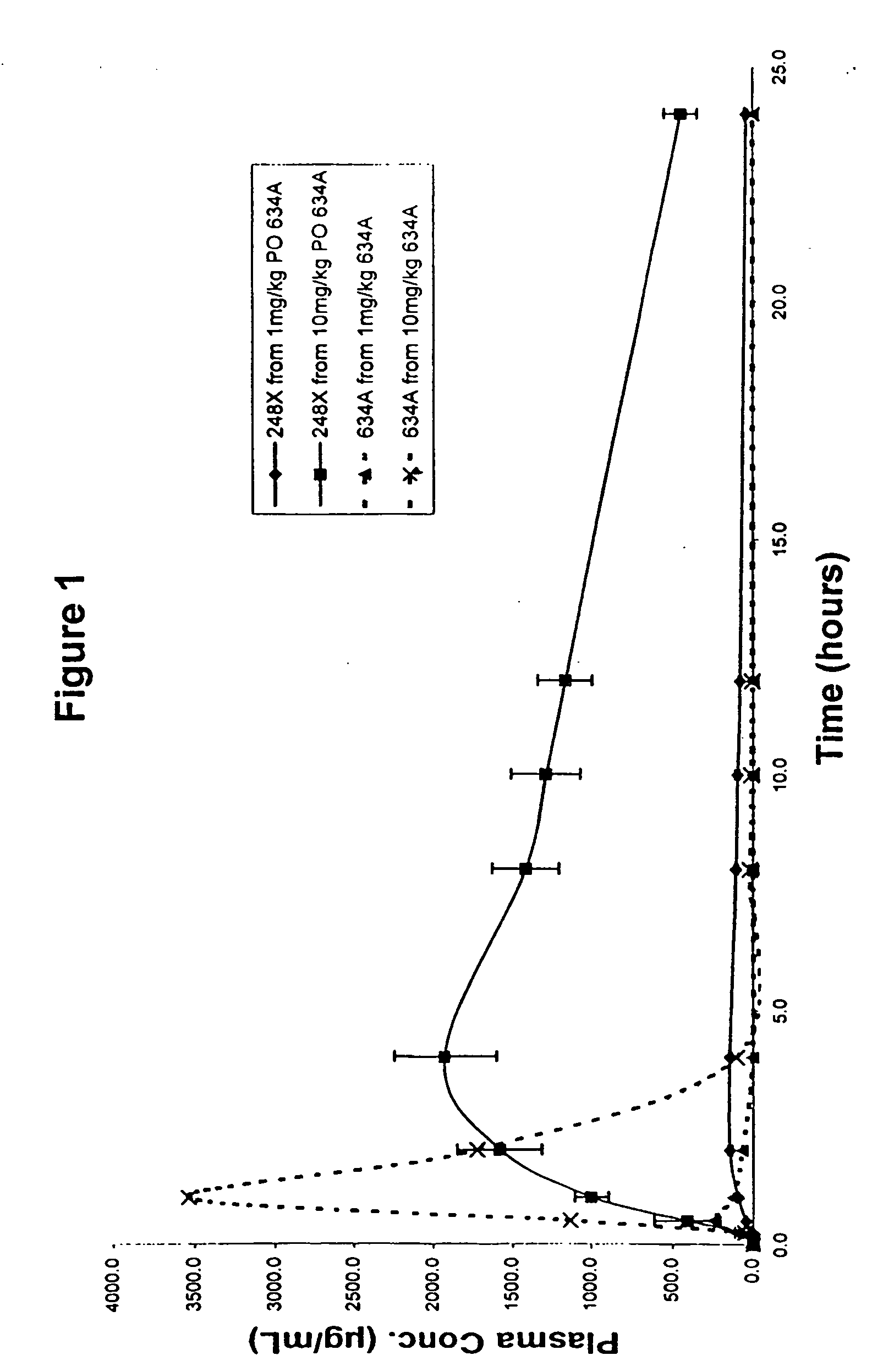
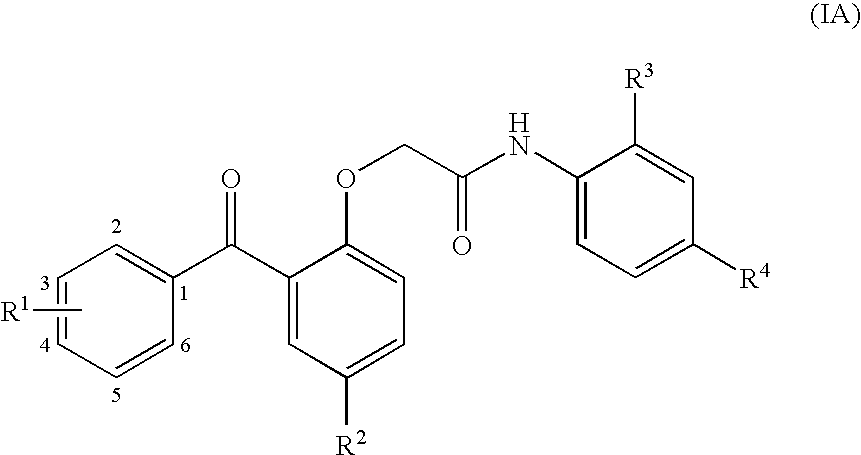
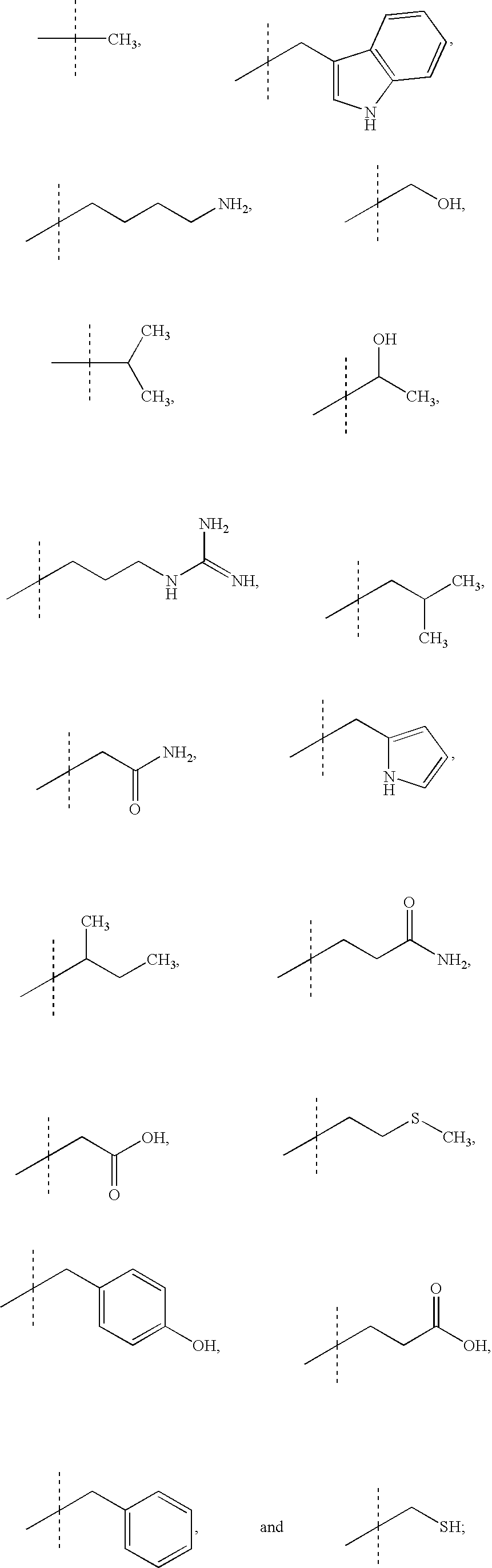
Benzophenones as inhibitors of reverse transcriptase
a reverse transcriptase and benzophenone technology, applied in the direction of capsule delivery, organic active ingredients, organic chemistry, etc., can solve the problem of reducing the sensitivity of other reverse transcriptas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
N-{4-[(acetylamino)sulfonyl]-2-methylphenyl}-2-[4-chloro-2-(3-cyano-5-methylbenzoyl)phenoxy]acetamide, Sodium Salt
[0308]
Step A:
[0309] Into a round-bottom flask were placed 2-aminotoluene-5-sulfonic acid (50.0 g, 267 mmol), and pyridine (300 mL). Acetic anhydride (38 mL, 403 mmol) was added dropwise from an addition funnel and the resulting mixture was allowed to stir for 2 h at rt. The solvents were removed under reduced pressure, leaving a brown solid. Several portions of ethyl alcohol were added to the solid and subsequently removed under reduced pressure, to afford a brown solid which was filtered and washed with several additional portions of ethyl alcohol and dried under vacuum (67.03 g, 81%). 1H NMR (DMSO-d6,) δ 2.08 (s, 3H), 2.22 (s, 3H), 7.39 (s, 2H), 7.45 (s, 1H), 8.02 (t, J=6 Hz, 2H), 8.53 (t, J=6 Hz, 1H), 8.92 (d, J=6 Hz, 2H), 9.31 (s, 1H).
Step B:
[0310] The compound from step A (67.03 g, 217 mmol) was added to a round-bottom flask containing 1N NaOH (225 mL) and t...
example 2
2-[4-chloro-2-(3-cyano-5-methylbenzoyl)phenoxy]-N-{4-[(isobutyrylamino)sulfonyl]-2-methylphenyl}acetamide, Sodium Salt
[0323]
Step A:
[0324] Following the procedure for the synthesis of 6 and using 12 (1 g, 5.4 mmol), isobutyric acid (0.6 mL, 5.9 mL) and fuming sulfuric acid (5 mL), 15 (0.67 g, 48%) was obtained as an off-white solid.
Step B:
[0325] Following the procedure for the synthesis of 13 and using 15 (0.2 g, 0.6 mmol), 16 (0.12 g, 33%) was obtained as a white solid. 1H NMR (DMSO-d6, 300 MHz) δ 1.9 (d, 6H), 2.2 (s, 3H), 2.4 (s, 3H), 2.3-2.5 (m, 1H), 4.8 (s, 2H), 7.2 (d, 1H), 7.5 (s, 1H), 7.6-7.8 (m, 4H), 7.8-8 (m, 3H), 9.4 (s, 1H), 12 (s,.67 (s, 2H).
Step C:
[0326] Following the procedure used for the synthesis of 1 and using 16 (0.12 g, 0.2 mmol) and 1 equivalent of NaOH, 14 (0.13 g, 100%) was obtained as a white fluffy solid. 1H NMR (DMSO-d6, 300 MHz) δ 1.9 (d, 6H), 2.2 (s, 3H), 2.4 (s, 3H), 2.3-2.5 (m, 1H), 4.8 (s, 2H), 7.2 (d, 1H), 7.5 (s, 1H), 7.6-7.8 (m, 4H), 7.8-8...
example 3
2-[4-chloro-2-(3-cyano-5-methylbenzoyl)phenoxy]-N-{2-methyl-4-[(propionylamino)sulfonyl]phenyl}acetamide, Sodium Salt
[0327]
Step A:
[0328] Propionic acid (Aldrich, 1.87 mL, 25 mmol) was added dropwise to fuming sulfuric acid (5 mL) in a round bottomed flask cooled in a water bath. The solution was stirred for 1 h at rt then 4-amino-3-methylbenzenesulfonamide (5) (3.26 g, 17.4 mmol) was added in one portion. The reaction was heated to 40° C. and stirred for an additional 4 h. The syrup was poured into ice water and the pH was adjusted to 4-5 with concentrated ammonium hydroxide. The resulting suspension was stirred vigorously for 2 h. The suspension was filtered and the solid was further purified on silica gel by flash chromatography using 95:5 methylene chloride (CH2Cl2):methanol (CH3OH) as eluant to afford an orange oil (1.86 g, crude). The crude oil was triturated with diethyl ether and the resulting solid was filtered and air-dried to afford 18 as a pale yellow solid (1.46 g, 3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com