Method of coating implantable medical devices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
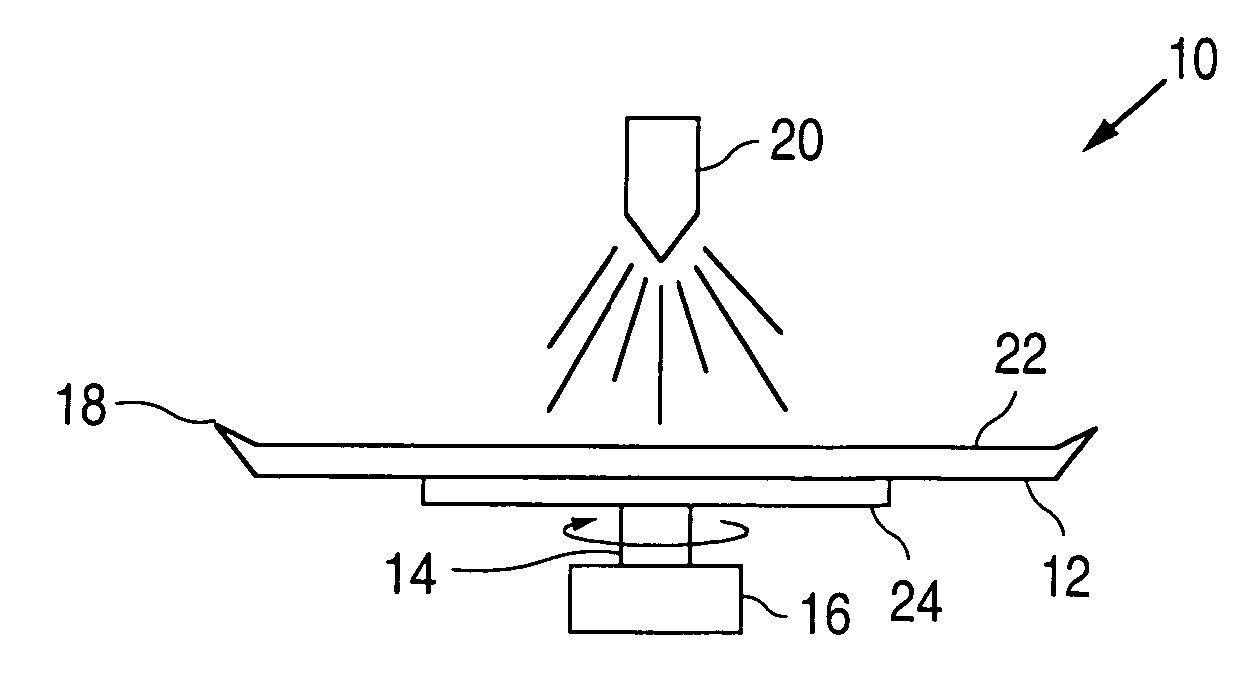
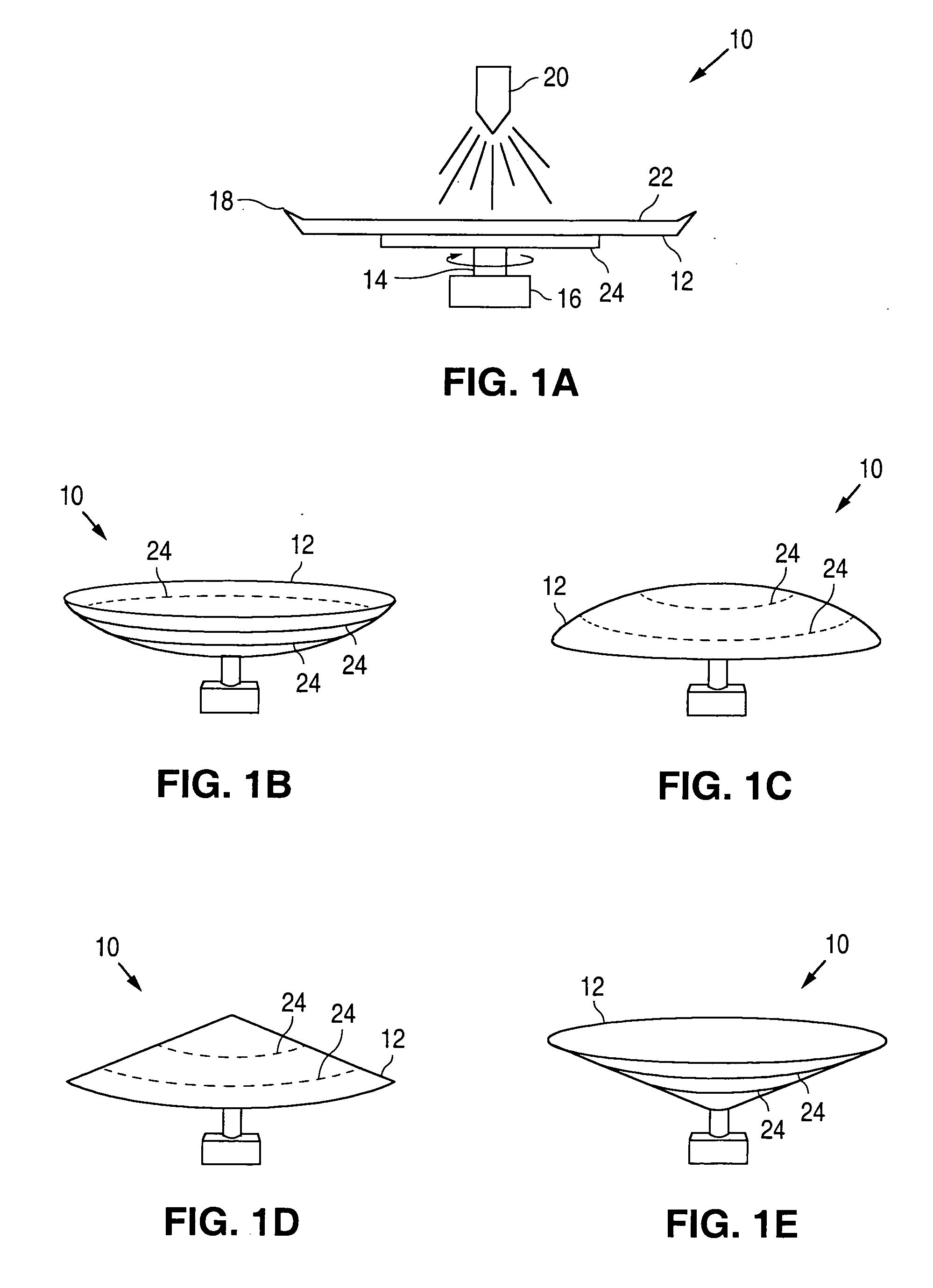
[0018]FIGS. 1A-1E illustrate embodiments of an apparatus 10 for coating medical devices, such as stents. Apparatus 10 can include a disk member 12 mounted on a shaft 14. The shaft 14, in turn, can be connected to a motor 16 for rotating the disk member 12 in a clockwise or counterclockwise direction. The disk member 12 can be flat (FIG. 1A), concave (FIG. 1B), convex (FIG. 1C) or conical (FIGS. 1D and 1E) in shape. The disk member 12 can optionally include a lip 18 disposed about the periphery thereof. The lip 18 can extend in an upwardly direction, towards a nozzle 20.
[0019] The disk member 12 can be made from any suitable material or can be coated with the desired material so as to minimize the ability of the composition to adhere to a surface 22 of the disk member 12 on which the composition is applied via the nozzle 20. One suitable non-stick surface 22 can be TEFLON. A temperature adjustor 24 can also be provided for adjusting the temperature of the composition during the coat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Percent by volume | aaaaa | aaaaa |
| Percent by volume | aaaaa | aaaaa |
| Percent by volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com