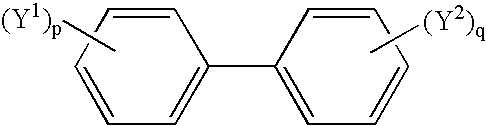
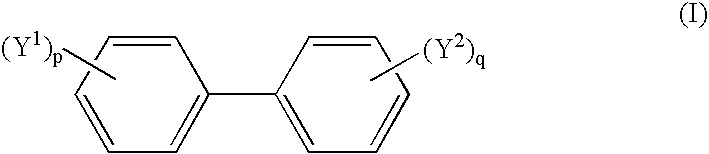
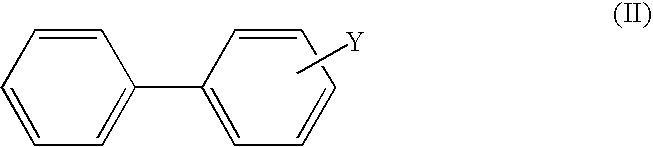Non-aqueous electrolytic solution and lithium secondary battery
a lithium secondary battery, non-aqueous electrolytic technology, applied in the direction of non-aqueous electrolyte cells, cell components, electrochemical generators, etc., can solve the problems of unsatisfactory battery performance of conventional lithium secondary batteries, and achieve high battery performance, high electric capacity, and high cycling performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0027] 1) Preparation of Electrolytic Solution
[0028] A non-aqueous solvent, i.e., a mixture (1:2, volume ratio) of propylene carbonate (PC) and diethyl carbonate (DEC), was prepared. Subsequently, LiPF6 was dissolved in the non-aqueous solvent to give a 1M concentration solution. Further, biphenyl was added to give a 0.1 wt. % solution. Thus, an electrolytic solution was prepared.
[0029] 2) Preparation of Lithium Secondary Battery and Measurement of Battery Performances
[0030] LiCoO2 (positive electrode active material, 80 wt. %), acetylene black (electro-conductive material, 10 wt. %), and poly(vinylidene fluoride) (binder, 10 wt. %) were mixed. The resulting mixture was diluted with 1-methyl-2-pyrrolidone. Thus produced positive electrode composition was coated on aluminum foil, dried, molded under pressure, and heated to give a positive electrode.
[0031] Natural graphite (d002=0.3354, 90 wt. %) and poly-(vinylidene fluoride) (binder, 10 wt. %) were mixed. The mixture was then di...
example 2
[0062] A secondary battery was prepared in the same manner as in Example 1, except that 0.1 wt. % of biphenyl was replaced with 0.05 wt. % of 4-methoxybiphenyl.
[0063] The prepared secondary battery was subjected to the 100 cycle charge-discharge procedure.
[0064] The initial discharge capacity was 1.03 (relative value based on 1 for that measured in a battery of Comparison Example 1 which contained no biphenyl in the electrolytic solution).
[0065] After the 100 cycle charge-discharge procedure, the discharge capacity was 90.8% of the initial discharge capacity.
example 3
[0066] A secondary battery was prepared in the same manner as in Example 1, except that 0.1 wt. % of biphenyl was replaced with 0.1 wt. % of 4-methoxybiphenyl.
[0067] The prepared secondary battery was subjected to the 100 cycle charge-discharge procedure.
[0068] The initial discharge capacity was 1.03 (relative value based on 1 for that measured in a battery of Comparison Example 1 which contained no biphenyl in the electrolytic solution).
[0069] After the 100 cycle charge-discharge procedure, the discharge capacity was 92.4% of the initial discharge capacity.
PUM
| Property | Measurement | Unit |
|---|---|---|
| operating voltage | aaaaa | aaaaa |
| weight % | aaaaa | aaaaa |
| operating voltage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com