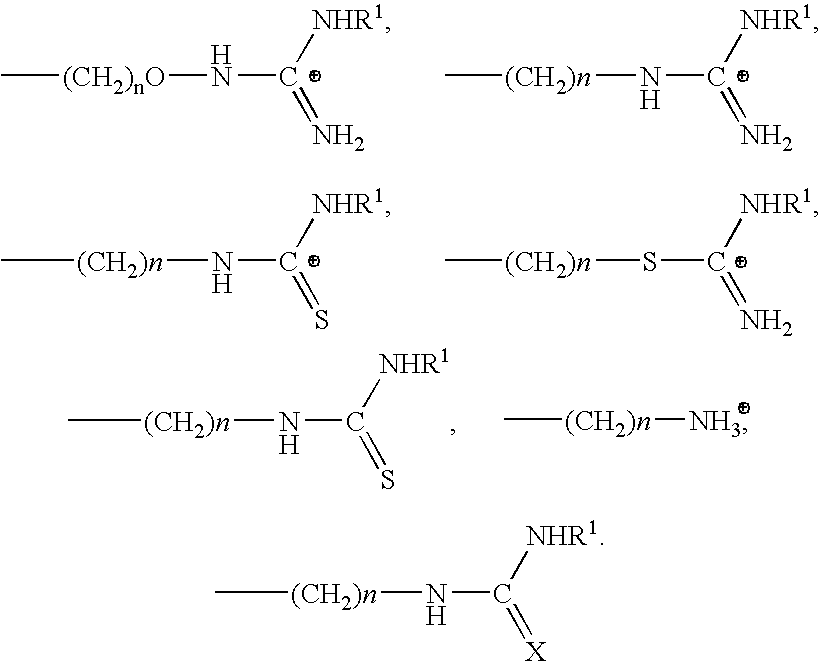
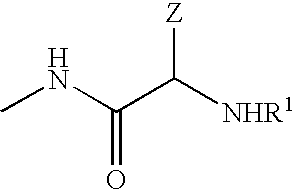
Cyclic agonists and antagonists of C5a receptors and G protein-coupled receptors
a technology of c5a receptors and g protein-coupled receptors, which is applied in the direction of peptide/protein ingredients, peptide sources, immunological disorders, etc., can solve the problems of enhancing inflammatory responses, lysis and cell death, and structure-function studies are extremely difficult to interpret, so as to facilitate the assembly of a “membrane attack complex, avoid stepwise amplification of proteolysis, and promote inflammation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Structure-Activity Relationship of C5a Agonists
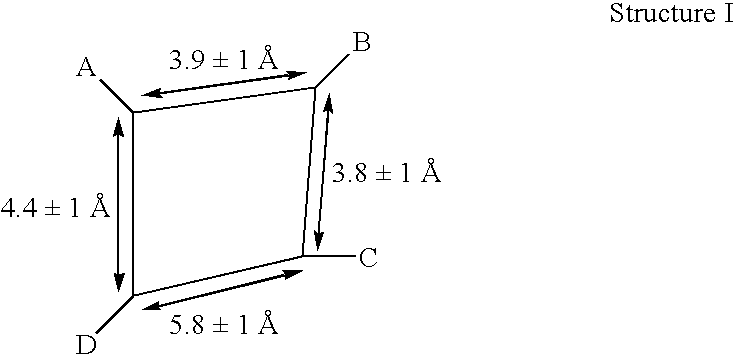
[0103] We have focussed on the C-terminal residues of C5a, in order to explore structure-activity relationships in the search for peptide sequences with potent agonists activity. Many of these peptides are full agonists relative to C5a, but have markedly lower potency (Sanderson et al, 1994, 1995; Finch et al, 1997) Our initial structure-activity investigations have been particularly informative. Mutating the decapeptide C-terminus of C5a (1, C5a65-74, ISHKDMQLGR) twice I65Y and H67F (eg. 2) led to enhancement of agonist potency by about 2 orders of magnitude. These results are summarised in Table 2. Analyses of Ramachandran plots and 2D NMR spectra for compound 2 suggested that certain structural features, namely a twisted “helix-like” backbone conformation for residues 65-69 and a β-turn for residues 71-74, might be responsible for activity. These preliminary results provided some insight to structural requirements for tight binding ...
example 2
NMR Structure of C5a Antagonist
[0107] We used two dimensional nuclear magnetic resonance spectroscopy to determine the three dimensional structure of 7 and found that while there is no discernible structure in water, there is evidence of a stable gamma-turn structure in dimethylsulfoxide.
[0108] The 1D 1H-NMR spectrum of peptide 7 in d6-DMSO at 24° C. shows 4 distinct resonances for amide-NH protons, as summarized in Table 3. To establish their possible involvement in intramolecular hydrogen bonds, a deuterium exchange experiment was performed by adding a 10-old excess of D2O to the solution. Two of the amide-NH doublets disappeared immediately, along with resonances attributable to the N-terminal methylamine protons. However, the other two amide NH resonances, as well as a broad resonance at approximately 8.05 ppm, persisted for up to 6.5 hours (FIG. 2). These three slowly-exchanging protons are assigned to the amide NHs of Trp and d-Cha and the side chi amine of Lys, the slow exc...
example 3
Structure-Activity Relationships In Vitro
[0114] We also examined the receptor-binding and antagonist activity of the hexapeptide 7 for comparison with our new compounds. The previous report by Konteatis et al (1994) concerned the ability of 7 to compete with C5a binding to receptors on isolated PMN membranes (IC50 70 nM), which is not necessarily physiologically relevant. We examined competition between 7 and C5a using intact PMN cells, and found that, under these conditions, 7 binds with much lower receptor affinity of IC50 1.8 μM. We confirmed that 7 is a full antagonist with no agonist properties. These results are summarized in FIG. 5a and Table 4. The relative affinity (ratio) of 7 for the C5aR in intact PMNs in our assays was similar to that previously reported for isolated PMN membranes.
[0115] We have also found that 7 shows antagonist activity against both C5a (FIG. 5b) and a C-terminal agonist decapeptide analogue 4 (YSFKPMPLaR) (Finch et al, 1997) of the C-terminus C5a65...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Structure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com