Medical compositions containing ghrelin
a technology of ghrelin and composition, which is applied in the field of pharmaceutical compositions containing ghrelin, can solve the problems of compound degradation in the formulation process or in the storage process thereafter, the stability of these peptides for development as medicines has never been studied, and it is difficult to develop an oral administrable composition containing these peptides or proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Structural Analysis of the Degradation Products of the Ghrelins
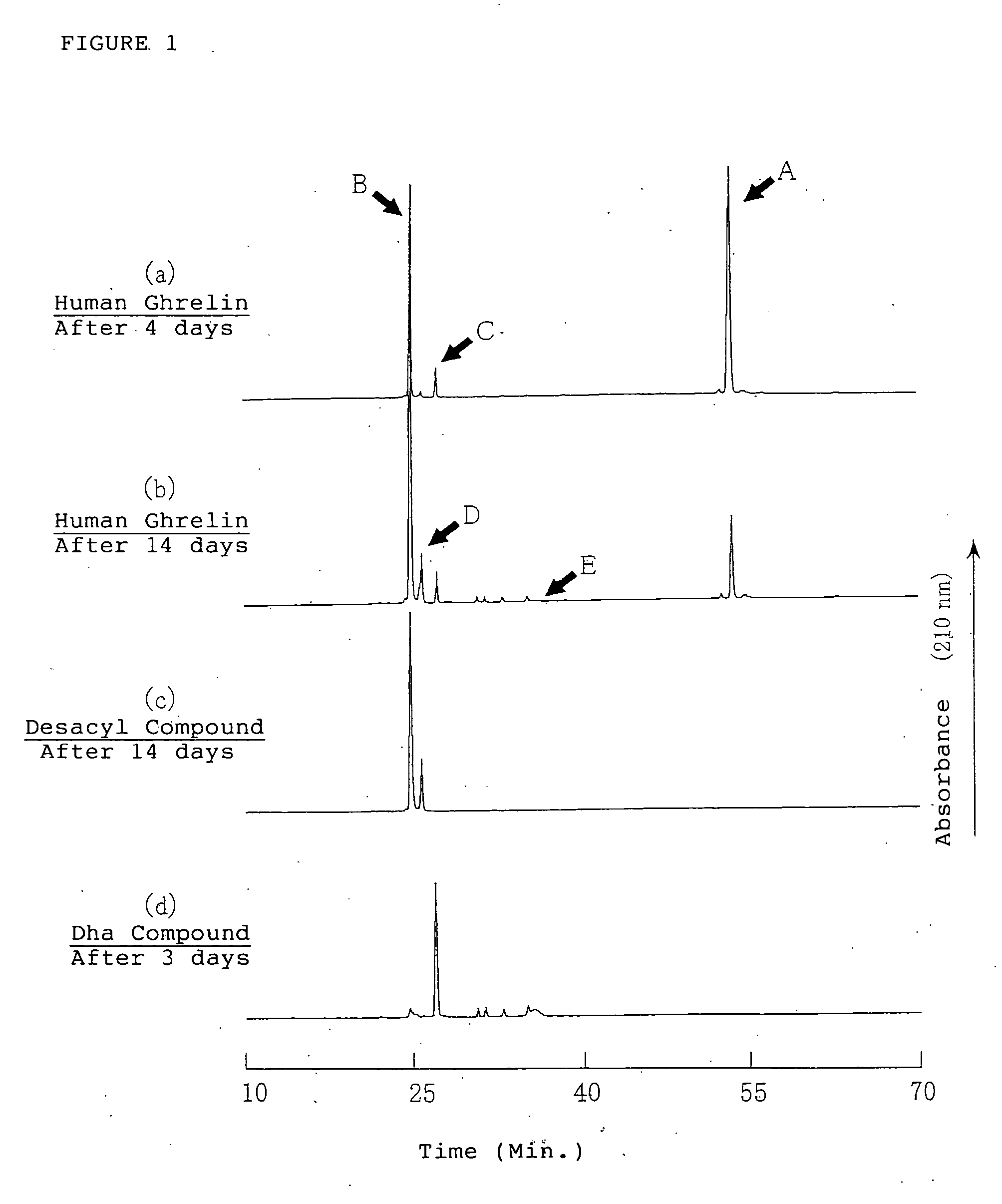
[0142] It is necessary to know the degradation reaction of the ghrelins in the aqueous solution to secure the stability of ghrelin in the aqueous solution. Therefore, the degradation process of ghrelin was estimated by the structural analysis of the degradation products of the ghrelins by using human ghrelin, which is one of the ghrelins.
[0143] The aqueous solution containing about 0.15 μmol / mL (0.5 mg / mL) of human ghrelin was obtained by dissolving about 5.0 μmol (17 mg) of human ghrelin in Britton-Robinson buffer solution (pH 7.0: adjusted by 0.04M of phosphate / acetic acid / boric acid solution) and 0.2M sodium hydroxide aqueous solution. The obtained solution was filled in brownish glass ampoules and the ampoules were sealed with fire. The each ampoules were stored at 40±1° C. for 4 and 14 days. The degradation products in the aqueous solutions after storage were detected by HPLC method and the results were shown in F...
example 2
Stability of the Ghrelins in Buffer Solutions having Various Kinds of pH Value (Stability Test 1)
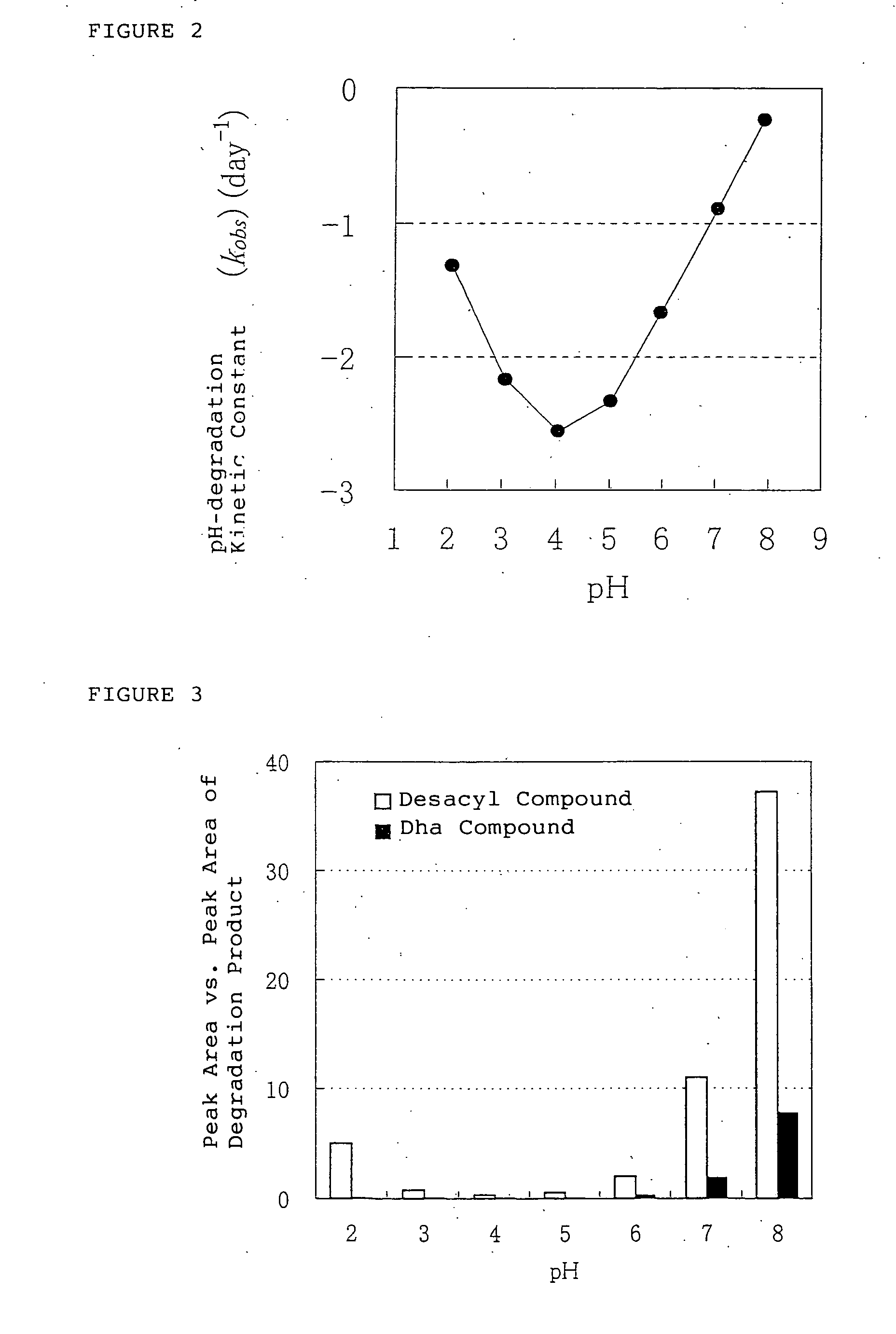
[0159] The influence of pH value of the solution containing the ghrelins was conducted using human ghrelin, which is one of the ghrelins.
[0160] Human ghrelin was dissolved in the following aqueous solutions in the concentration of about 0.15 μmol / mL (0.5 mg / mL).
[0161] 0.1M HCl aqueous solution (pH: 1.1)
[0162] Mcllvain buffer solutions (pH: 2.0, 3.0, 4.0, 5.0, 6.0, 7.0, 8.0)
[0163] The pH was adjusted with 0.1M citric aqueous acid and 0.2M dibasic sodium phosphate aqueous solution.
[0164] Each solution were stored at 25±2° C. for 8, 24, 48 and 72 hours respectively, and the obtained solutions were detected by HPLC analysis in comparison to the solutions before storage. The peak area ratio of human ghrelin, desacyl compound and Dha compound to the total area were calculated. No significant changes of pH in solution before storage and after storage were observed. The results were shown ...
example 3
Stability of the Ghrelins in Buffer Solutions having Various Kinds of pH Value (Stability Test 2)
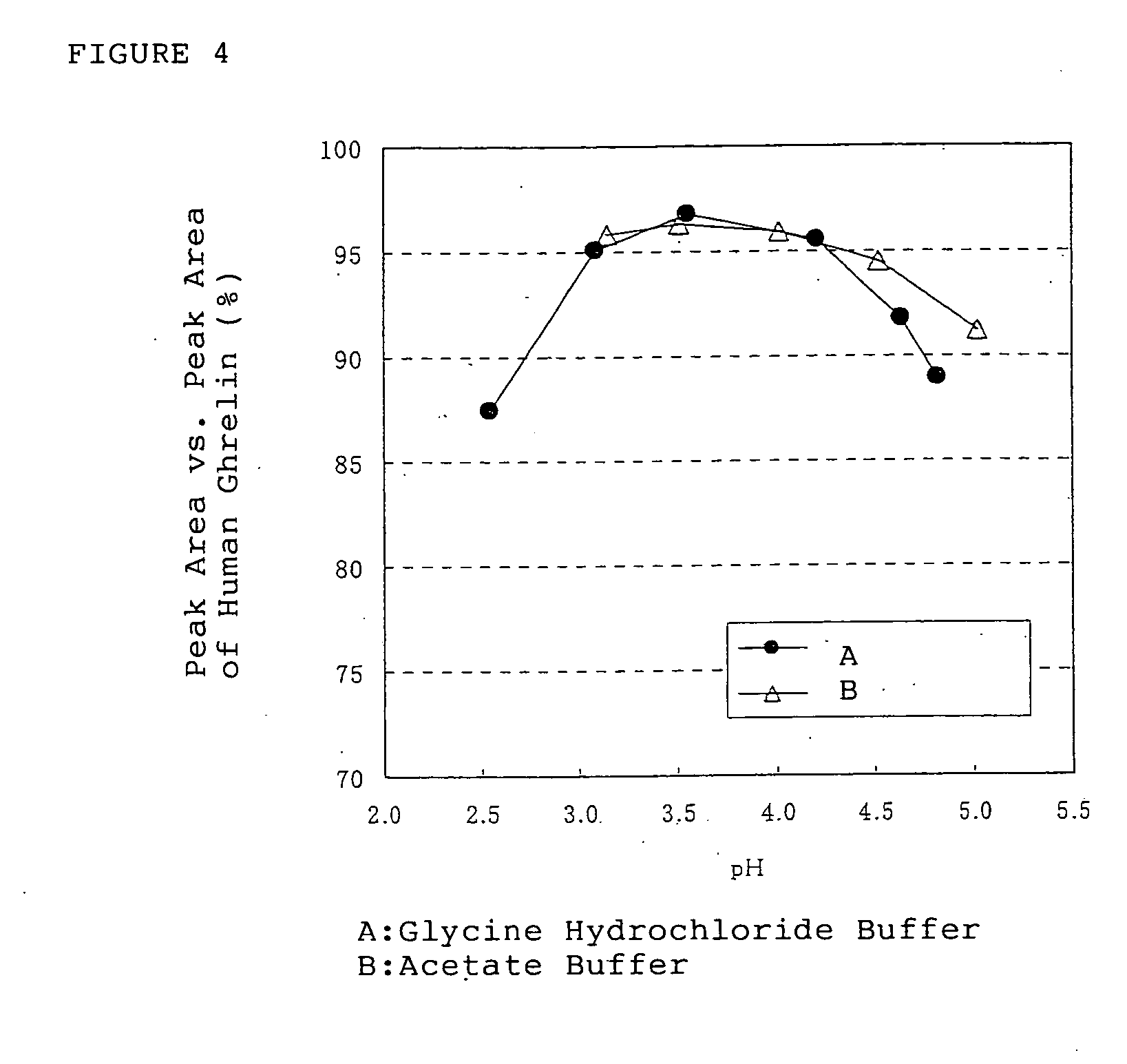
[0167] Using different buffer solution from the buffer solution of the Example 2, the stability of the ghrelins was conducted in the buffer solution having various kinds of pH value.
[0168] Human ghrelin, which is one of the ghrelins, was dissolved in the Britton-Robinson buffer solutions which is adjusted by combining 0.04M phosphoric acid-acetic acid-boric acid aqueous solution and 0.2M sodium hydrate aqueous solution in appropriate ratio, having pH of 2.1, 3.1, 4.0, 5.0, 6.0, 7.0 and 7.9 to obtain an aqueous solution containing human ghrelin in the concentration of about 0.15 μmol / mL (0.5 mg / mL). The obtained solution was filled in brownish glass ampoules and the ampoules were sealed with fire. For the calculation of kinetic constant, the certain degrees of degradation products have to be occurred in the aqueous solution of the ghrelins, and therefore, the ampoules were stored at 40±...
PUM
| Property | Measurement | Unit |
|---|---|---|
| flow rate | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| retention time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com