Coated endovascular stent
a stent and endovascular technology, applied in the field of endovascular stents, can solve the problems of affecting the shelf life of such devices, and thrombogenic effects of flow, so as to facilitate the subsequent loading of heparin and prolong the shelf life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
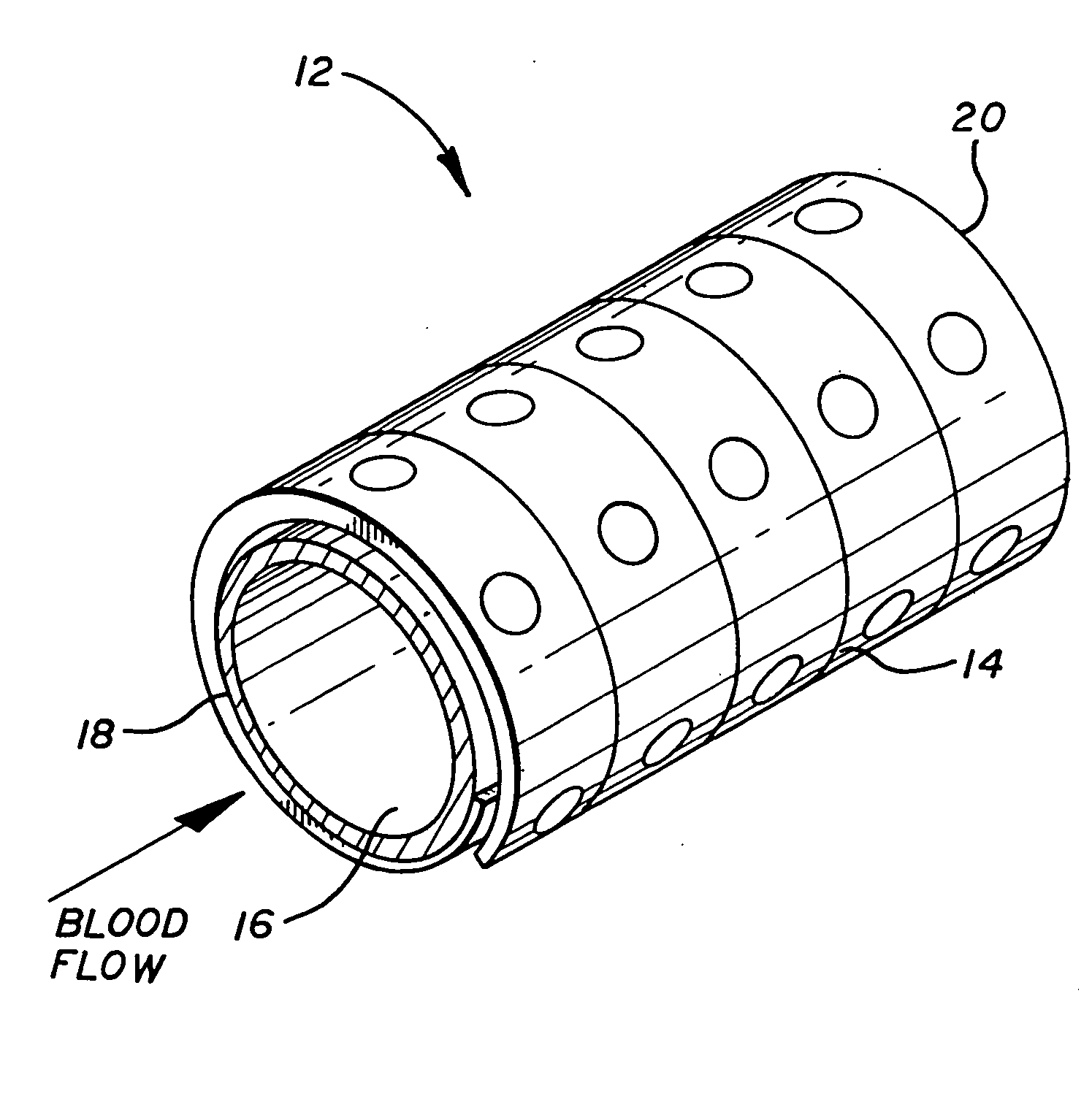
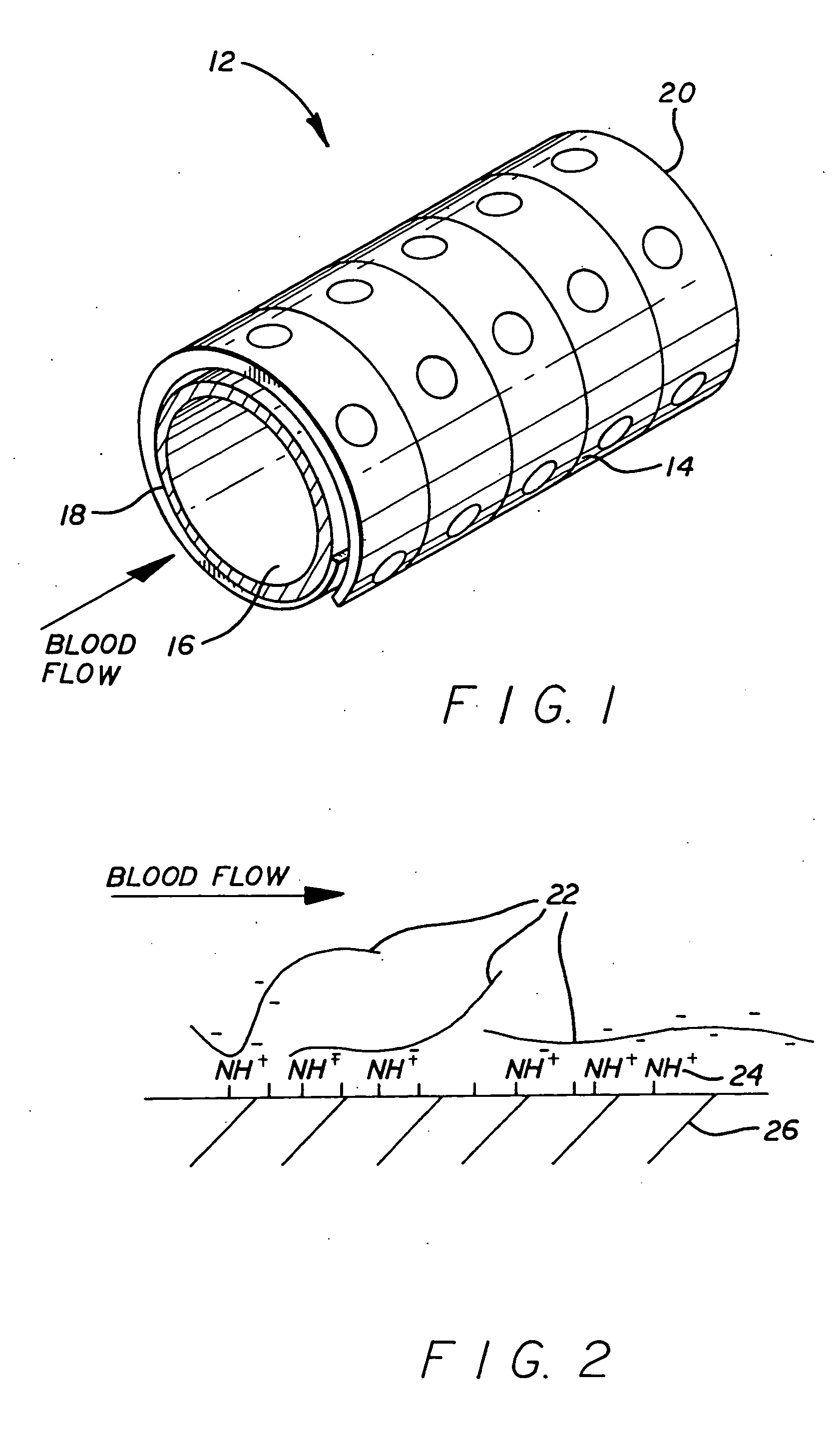
[0015] A wide variety of different stent configurations have been devised to address various issues inherent in their use and function. Additionally, various materials have been employed in their construction including metals and polymers. Both the degree of turbulence caused by a particular stent configuration when subjected to blood flow as well as the material from which it is constructed affects the degree of thrombogenicity associated with a particular stent device. The present invention provides a coating for such stents to which heparin becomes attached and thus serves to reduce or eliminate thrombosis formation. Moreover, the stent's coating allows the heparin to be loaded thereon immediately before the implantation procedure or after the stent is in place.
[0016] Critical requirements for the coating of the present invention include that it adheres to the stent surface and that it has functional groups that attract heparin and to which heparin bonds. Functional groups that ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| covalent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com