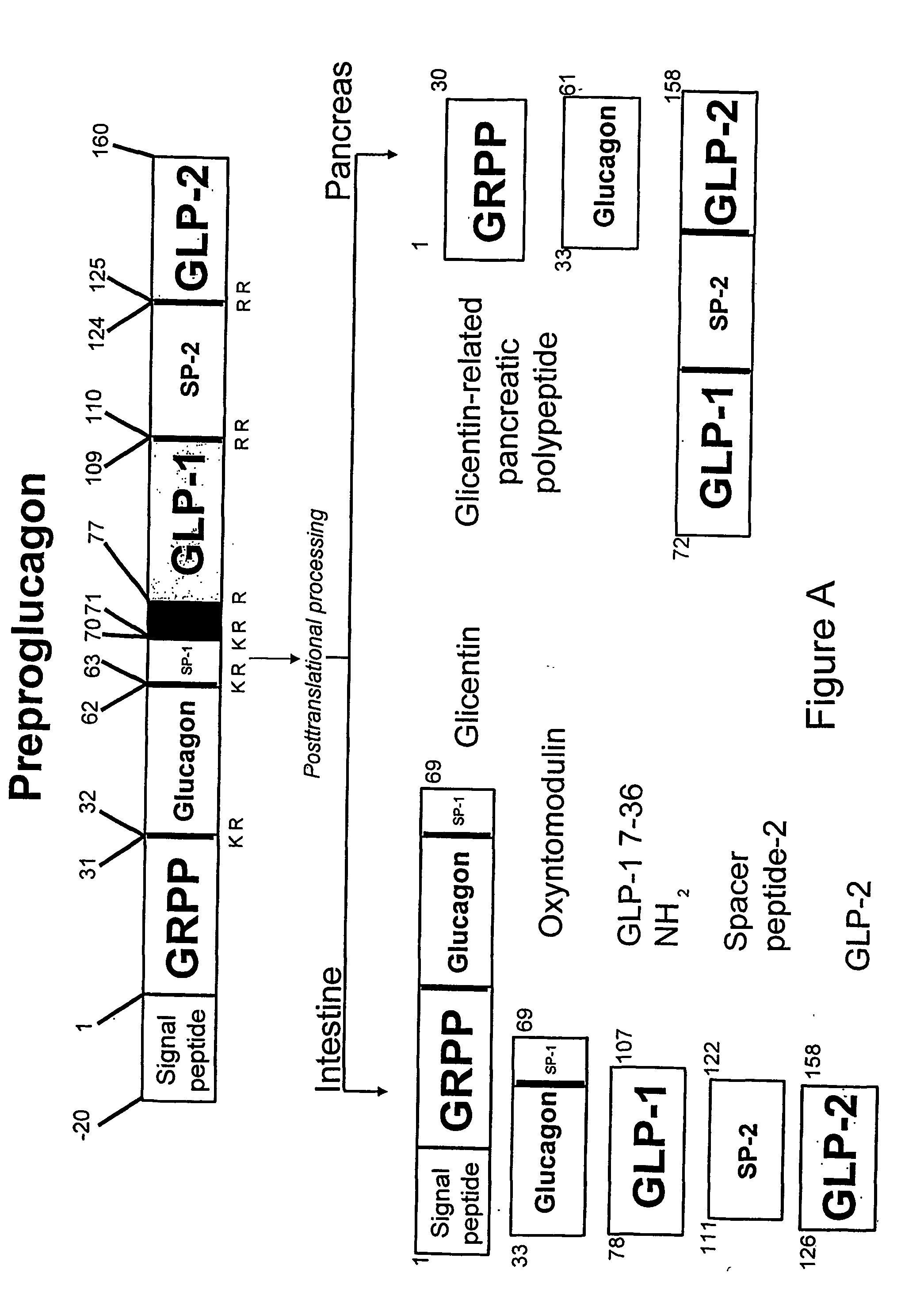
Modification of feeding behaviour
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
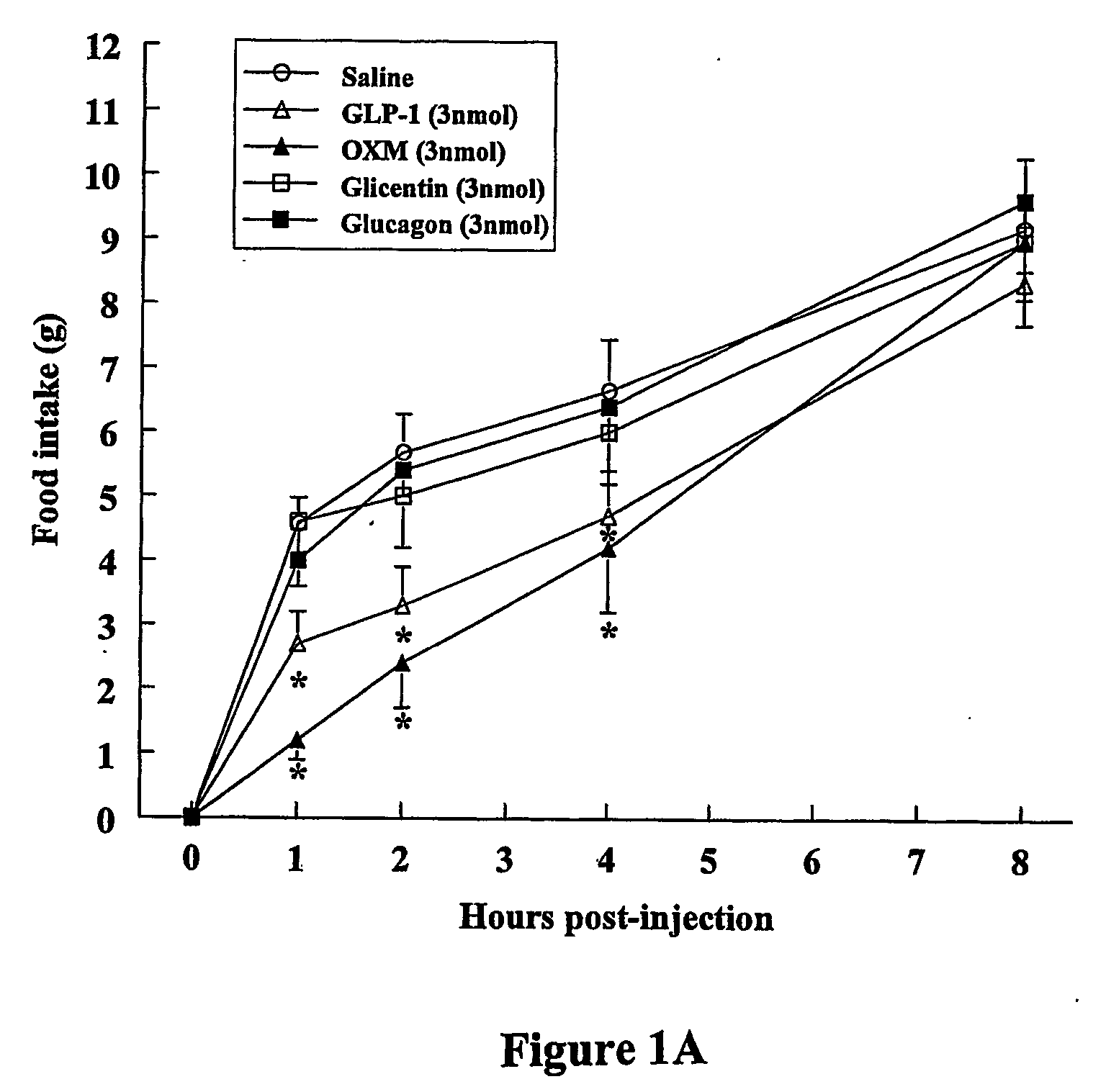
OXM Causes a Potent Decrease in Fasting-Induced Refeeding when Injected Both ICV and iPVN
Peptides and Chemicals
[0111] GLP-1, glicentin, glucagon, and SP-1 were purchased from Peninsula Laboratories, Inc. (St. Helens, UK). OXM was purchased from IAF BioChem Pharma (Laval, Canada). Exendin-4 and exendin-(9-39) were synthesised at Medical Research Council, Hemostasis Unit, Clinical Sciences Center, Hammersmith Hospital, London, UK using F-moc chemistry on an 396 MPS peptide synthesiser (Advanced ChemTech, Inc.) and purified by reverse phase HPLC on a C8 column (Phenomex, Macclesfield, UK). The correct molecular weight was confirmed by mass spectrometry. All chemicals were purchases from Merck & Co. (Lutterworth, Leicester, UK) unless otherwise stated.
Animals
[0112] Adult male Wistar rats (ICSM, Hammersmith Hospital) were maintained in individual cages under controlled conditions of temperature (21-23° C.) and light (12 h of light, 12 h of darkness) with ad libitum access to food ...
example 2
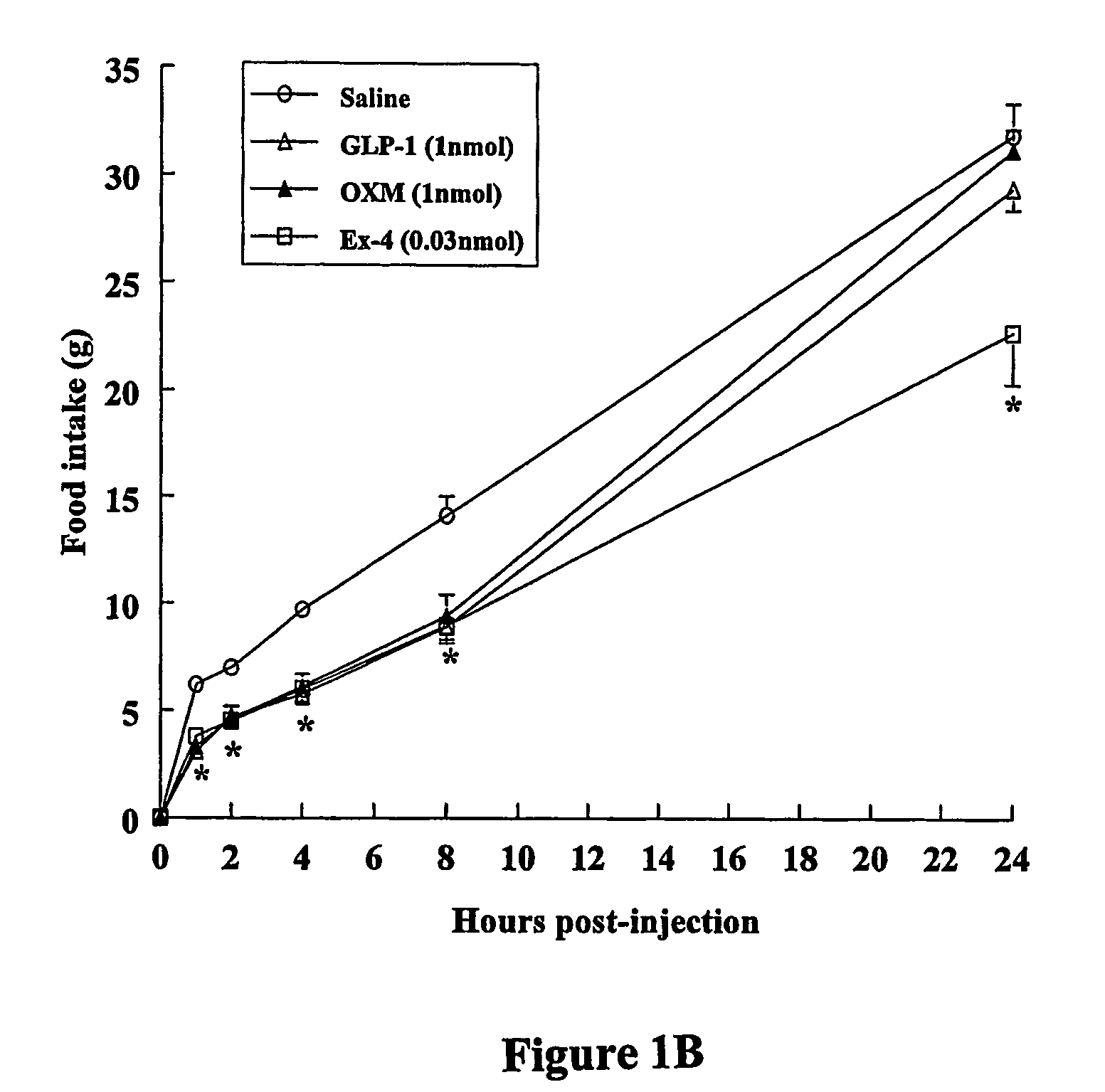
Peripheral Administration of OXM Also Reduces Food Intake and Body Weight Gain.
Peptides and Chemicals
[0139] OXM was purchased from IAF BioChem Pharma (Laval, Canada). GLP-1 was purchased from Peninsula Laboratories Inc. (St. Helens, UK). Exendin 9-39 was synthesised at Medical Research Council, Hemostasis Unit, Clinical Sciences Centre, Hammersmith Hospital, London, UK using F-moc chemistry on a 396 MPS peptide synthesizer (Advanced ChemTech Inc., Louisville, Ky.) and purified by reverse phase HPLC on a C8 column (Phenomex, Macclesfield, UK), using a gradient of acetonitrile on 0.1% trifluoroacetic acid. Correct molecular weight was confirmed by mass spectrometry. All chemicals were purchases from Merck Eurolab Ltd. (Lutterworth, Leicestershire, UK), unless otherwise stated.
Animals
[0140] Adult male Wistar rats (180-200 g) were maintained in individual cages under controlled conditions of temperature (21-23° C.) and light (12 hours light, 12 hours dark) with ad libitum access ...
example 3
Investigation of the Effect of OXM Infusion on Food Intake in Human Subjects
Methods
Study 1
[0169] The study design was a double-blind placebo-controlled crossover, see FIG. 12. 13 healthy volunteers (age 27±2 yrs; BMI 25.3±0.7 kg−2) received a 90 minute intravenous infusion of OXM (3.0 pmol / kg / min) and an infusion of saline ≧1 week apart, in random order. OXM was dissolved in saline containing haemaccel (5% by volume) to reduce adsorption to the syringe and tubing. Volunteers completed a food diary for three days prior to each infusion and for the subsequent 24 hours. Subjects were instructed to follow a similar diet on the days preceding each infusion. They consumed an identical meal (of their choice) on the night before each infusion and fasted from 9 pm.
[0170] On each study day intravenous cannulae were inserted bilaterally into arm veins, one for administration of the infusion, while the other was used for blood-sampling. Subjects were attached to a cardiac monitor and blo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com