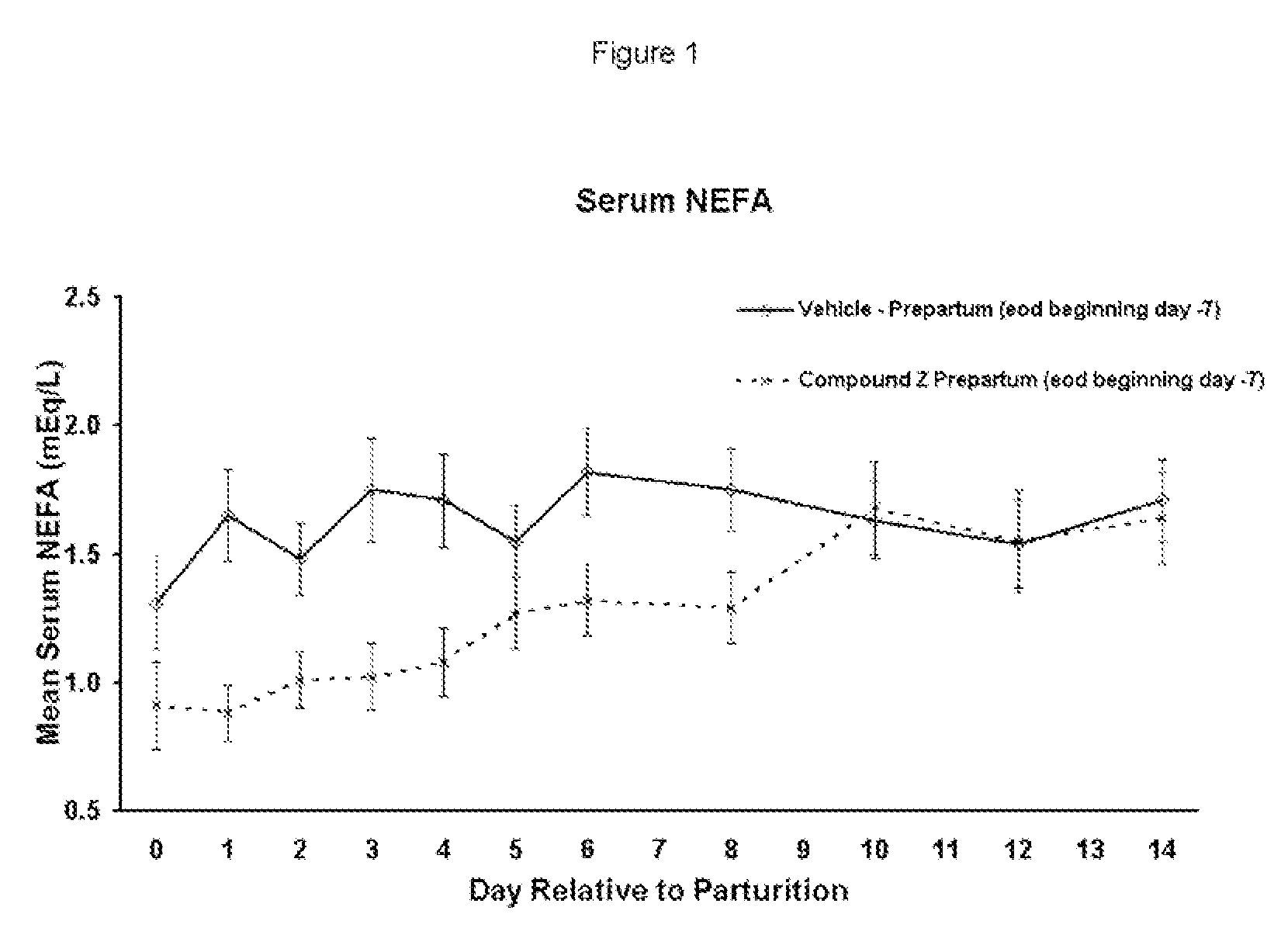
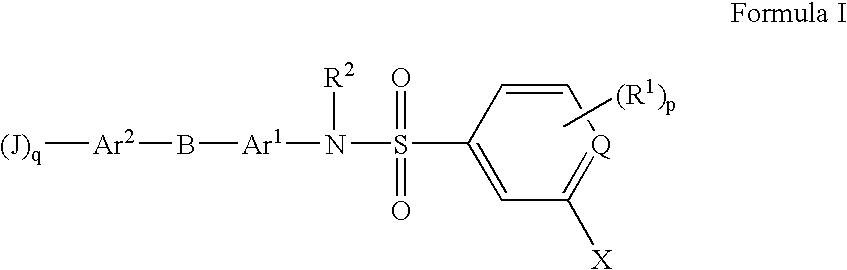
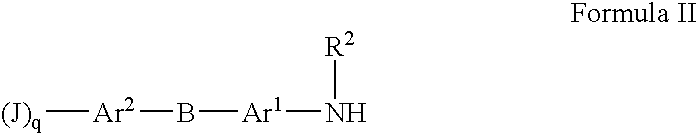
Substituted Heteroaryl- and Phenylsulfamoyl Compounds
a technology of phenylsulfamoyl and heteroaryland, which is applied in the field of substituting heteroaryland phenylsulfamoylcompounds, can solve the problems of limiting the use of clinically available hypoglycemic agents, large segments of western populations are now realized to be at particularly high risk, and achieves the reduction of ketone body levels, milk quality, and milk quality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
5-(4-Benzyloxy-phenylsulfamoyl)-2-methyl-benzoic acid
[0467]
[0468] To a solution of 5-chlorosulfonyl-2-methylbenzoic acid (200 mg, 0.85 mmol) and p-benzyloxyaniline (187 mg, 0.94 mmol) in 6 ml acetone and 3 ml dimethylformamide was added a solution of sodium bicarbonate (215 mg, 0.56 mmol) in 2 ml water. The resulting mixture was stirred overnight at room temperature. The acetone was then removed under reduced pressure and the residual mixture gas partitioned between 25 ml 1N aqueous hydrochloric acid solution and 25 ml ethyl acetate. The aqueous phase was separated and extracted with 2×25 ml ethyl acetate. The combined ethyl acetate extracts were dried (anhydrous sodium sulfate) and concentrated under reduced pressure. The residue was purified by flash column chromatography (silica gel, 15 gm) eluting with 9:1 chloroform / methanol to yield a white solid (154 mg). The solid was triturated with dichloromethane to yield the title compound (93 mg, 28% yield) as a white solid. MS: 395.6 ...
example 2
2-Methyl-5-[4-(6-methyl-benzothiazol-2-yl)-phenylsulfamoyl]benzoic acid
[0470]
[0471] 8% yield. MS: 439.4 (M+1) 1H NMR (400 MHz, CD3OD): δ2.44 (s, 3H), 2.53 (s, 3H), 7.23 (m, 2H), 7.29 (m, 1H), 7.34 (m 1H), 7.72 (m, 1H), 7.75 (m, 1H), 7.80 (m, 1H), 7.88 (m, 2H), 8.19 (m, 1H).
example 3
2-Methyl-5-[4-(5-methyl-benzooxazol-2-yl)-phenylsulfamoyl]benzoic acid
[0472]
[0473] 4% yield. MS: 423.4 (M+1); 1H NMR (400 MHz, CD3OD); δ2.42 (s, 3H), 2.51 (s, 3H), 7.17 (m, 1H), 7.26 (m, 2H), 7.35(m, 1H), 7.44 (m, 2H), 7.56 (m, 1H) 8.0 (m, 2H), 8.15 (m, 1H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com