Antisense antiviral compound and method for treating influenza viral infection
an antiviral compound and influenza virus technology, applied in the field of antiviral oligonucleotide compounds, can solve the problems of significant mortality and morbidity, inability to protect against unexpected strains, and inability to vaccina
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Inhibition of Influenza A virus in Cell Culture with Phosphorodiamidate Morpholino Oligomers
[0171] Phosphorodiamidate Morpholino Oligomers (PMOs), designed to hybridize to various gene segments of influenza A virus, were evaluated for their ability to inhibit influenza virus production in Vero cell culture. The PMOs were conjugated to a short arginine-rich peptide (R5F2R4C) to facilitate entry into cells in culture. Vero cells were incubated with PMO compounds, inoculated with influenza A virus (strain PR8, H1N1), and viral titer determined by hemagglutinin assay (HA) and / or plaque-assay (CFU). The PMO compounds targeting the AUG translation start-sites of polymerase component PB1 and nuclear capsid protein (NP) (SEQ ID NOs: 10 and 11), the 5′ and 3′ ends of the NP gene (SEQ ID NOs:13 and 14) encoded by the viral RNA (i.e. vRNA) and the 3′ end of the NP gene (SEQ ID NO:15) encoded by the complementary RNA (cRNA) were very effective in reducing the titer of influenza virus by 1 to 3...
example 2
Effect of PMO Targeting the 3′-Terminus of NP vRNA on NP mRNA and cRNA Transcription
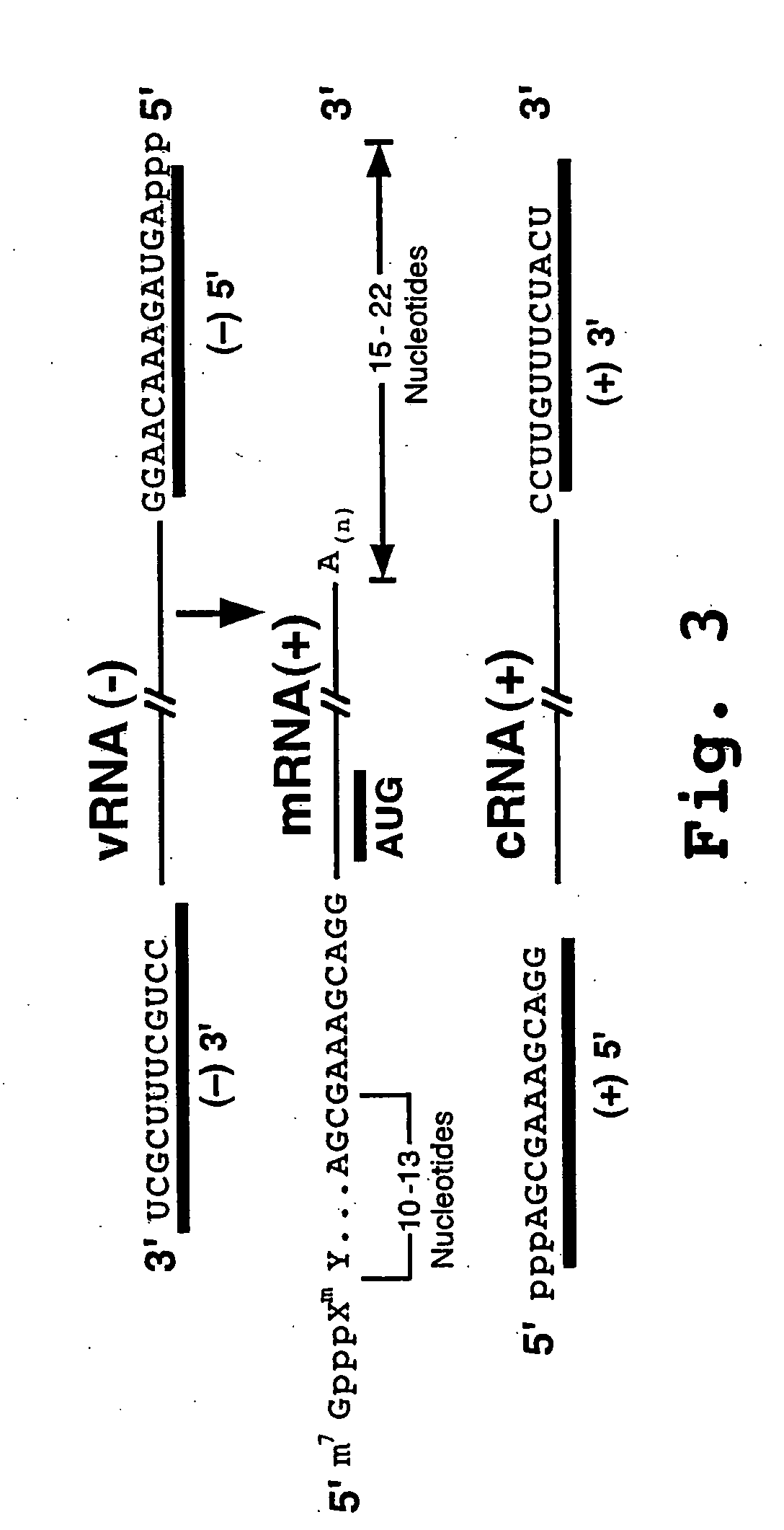
[0174] Quantitative RTPCR was used to determine the effect of one of the termini-targeted PMO, NP(−)3′ (SEQ ID NO:13) on the transcription of the NP vRNA segment into mRNA and cRNA species (i.e., see FIG. 4). The mRNA transcription product is positive-sense RNA whereas the cRNA is a negative-sense RNA. The NP(−)3′ PMO was incubated with Vero cells for 6 hours followed by influenza A virus infection at an MOI of 0.05. Three hours post-infection, RNA was isolated and RNA species specific reverse transcription (RT) was performed followed by quantitative PCR on the reaction product. FIG. 8 shows that the NP(−)3′ PMO (SEQ ID NO:13) that targets the 3′ end of the vRNA strongly suppressed the transcription of NP mRNA and cRNA.
example 3
Synergistic Inhibition of Influenza a Virus Replication in Cell Culture Using Combinations of Anti-influenza PMO
[0175] Combinations of some of the PMOs exhibited a synergistic antiviral effect. FIG. 9 shows the synergistic effect of various combinations of PMO that target the NP vRNA termini and the NP-AUG region. PMO treatment and influenza A virus infection were as described in Example 1. The plaque assay was used to measure virus replication. Three termini-targeted PMO, NP(−)32′, NP(−)5′, NP(+)3′ (SEQ ID NOs:13-15) and the NP-AUG PMO (SEQ ID NO:10) were mixed in various combinations as shown in FIG. 8. One combination, NP(+)3′ with NP(−)5′ did not produce antiviral activity as this pair of PMO are predicted to hybridize to each other. All the other PMO combinations demonstrated significant inhibition of influenza A viral replication.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tm | aaaaa | aaaaa |
| Tm | aaaaa | aaaaa |
| Tm | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com