Peptides antibodies directed thereagainst and methods using same for diagnosing and treating amyloid-associated diseases
a technology of amyloid-associated diseases and antibodies, which is applied in the direction of dipeptide ingredients, tripeptide ingredients, antibody ingredients, etc., can solve the problems of inability to demonstrate the in vivo effectiveness of such antisense molecules, and inability to demonstrate the in vivo effectiveness of such antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Alanine Scan of the hIAPP Basic Amylodogenic Unit—Rational and Peptide Synthesis
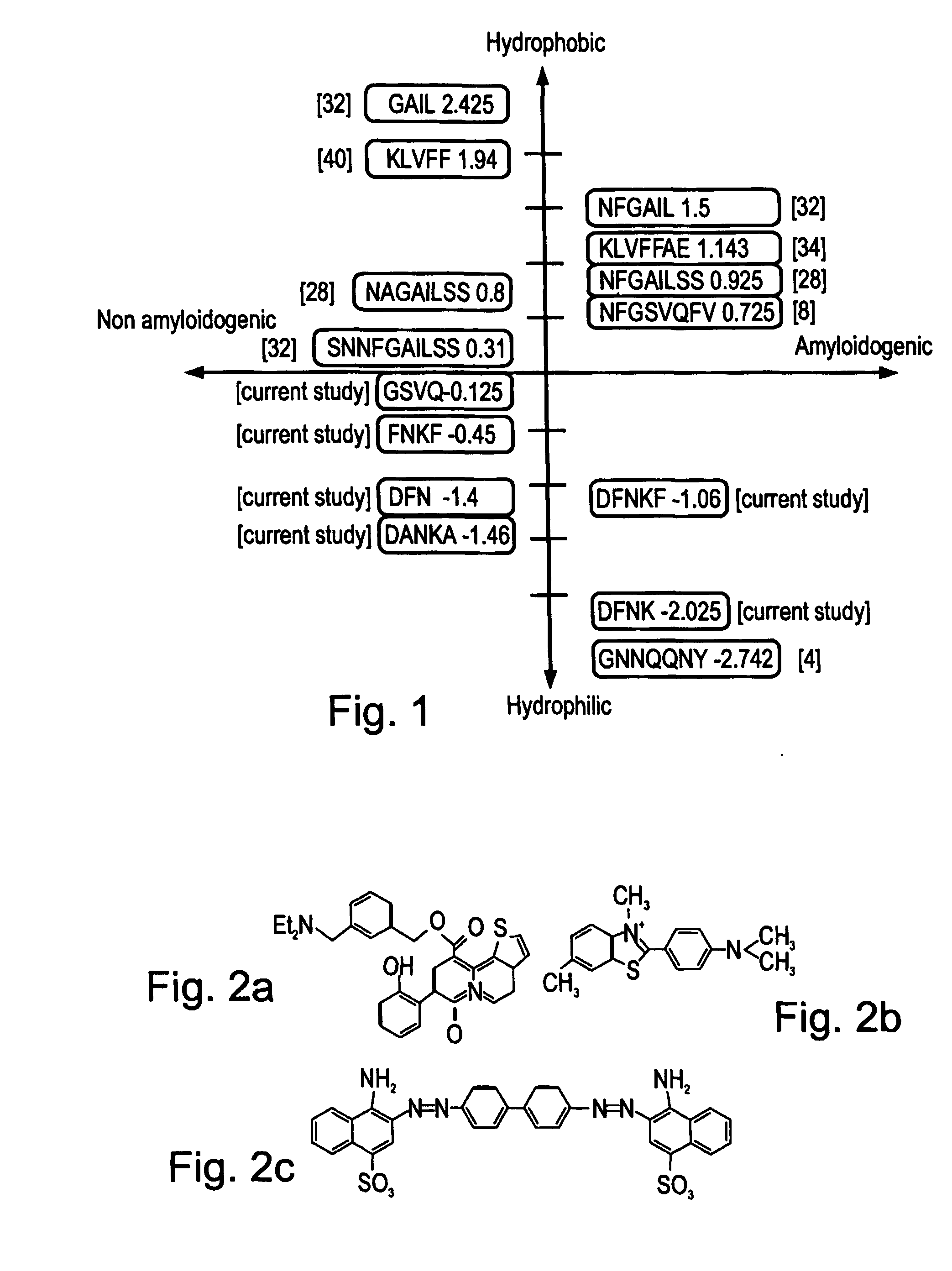
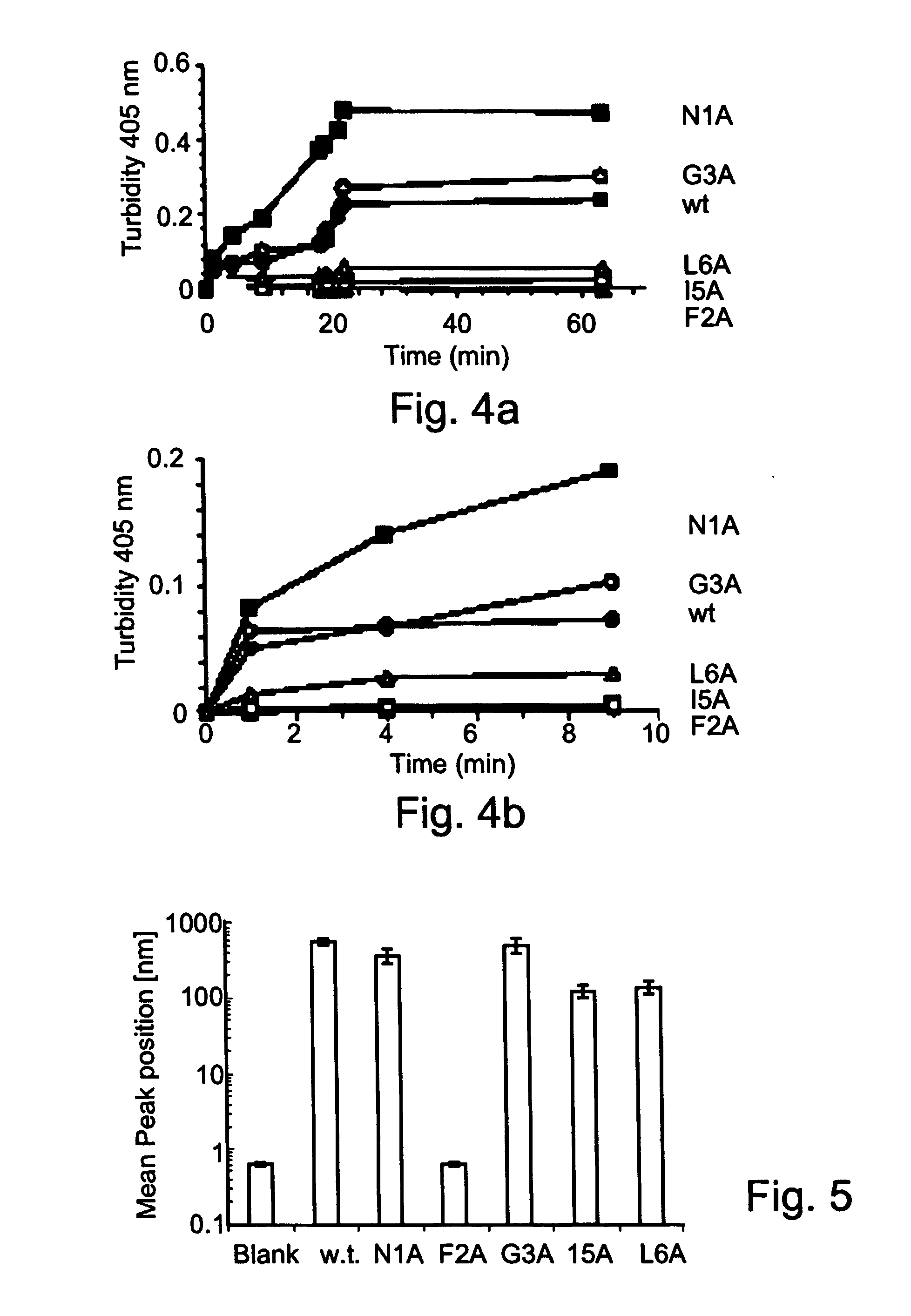
[0276] Pancreatic amyloid is found in more than 95% of type II diabetes patients. Pancreatic amyloid is formed by the aggregation of a 37 amino acid long islet amyloid polypeptide (IAPP, GenBank Accession No. gi:4557655), the cytotoxicity thereof being directly associated with the development of the disease. IAPP amyloid formation follows a nucleation-dependent polymerization process, which proceeds through conformational transition of soluble IAPP into aggregated β-sheets. Recently it has been shown that a hexapeptide (22-27) (NFGAIL, SEQ ID NO: 1) of IAPP, also termed as the “basic amyloidogenic unit” is sufficient for the formation of β-sheet-containing amyloid fibrils [Konstantinos et al. (2000) J. Mol. Biol. 295:1055-1071].
[0277] To gain further insight into the specific role of the residues that compose “the “basic amyloidogenic unit”, a systematic alanine scan was performed. Amino-acids were rep...
example 2
Kinetics of Aggregation of IAPP Peptide Fragment and Mutant Derivatives as Monitored by Turbidity Measurements
[0279] To study self-assembly of the IAPP peptide derived fragments, aggregation and insolubilization kinetics were monitored using turbidity measurements at 405 nm.
[0280] Kinetic aggregation assay—Fresh peptide stock solutions were prepared by dissolving lyophilized form of the peptides in DMSO, a disaggregating solvent, at a concentration of 100 mM. To avoid any pre-aggregation, fresh stock solutions were prepared prior to each and every experiment. Peptide stock solutions were diluted into assay buffer and plated in 96-well plates as follows: 2 μl of peptides stock solutions were added to 98 μl of 10 mM Tris pH 7.2, resulting in a 2 mM final concentration of the peptide in the presence of 2% DMSO. Turbidity data was measured at 405 nm. A buffer solution including 2% DMSO was used as a blank. Turbidity was measured at room temperature over several time points.
[0281] Res...
example 3
Measurement of Aggregate Mean Particle Size
[0283] While the turbidity assay provided an important estimate regarding the aggregation potential and kinetics of the various peptides, it did not provide information about the size of the actual aggregates formed. It will be appreciated that although the apparent hydrodynamic diameter of amyloid structures varies due to irregularity of the amyloid structure, it may still provide a clear indication about the order of magnitude of the structure formed and present a quantitative criterion for comparing the structures formed by the various peptides.
[0284] Therefore, the average size of the aggregates, formed by the various peptides, was determined using dynamic light scattering (DLS) experiments.
[0285] Method—Freshly prepared peptide stock solutions at a concentration of 10 mM were diluted in 10 mM Tris buffer pH 7.2 and further filtrated through a 0.2 μm filter to a final concentration of 100 μM peptide and 1% DMSO. Particle size measure...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| phi angle | aaaaa | aaaaa |
| Particle size measurement | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com