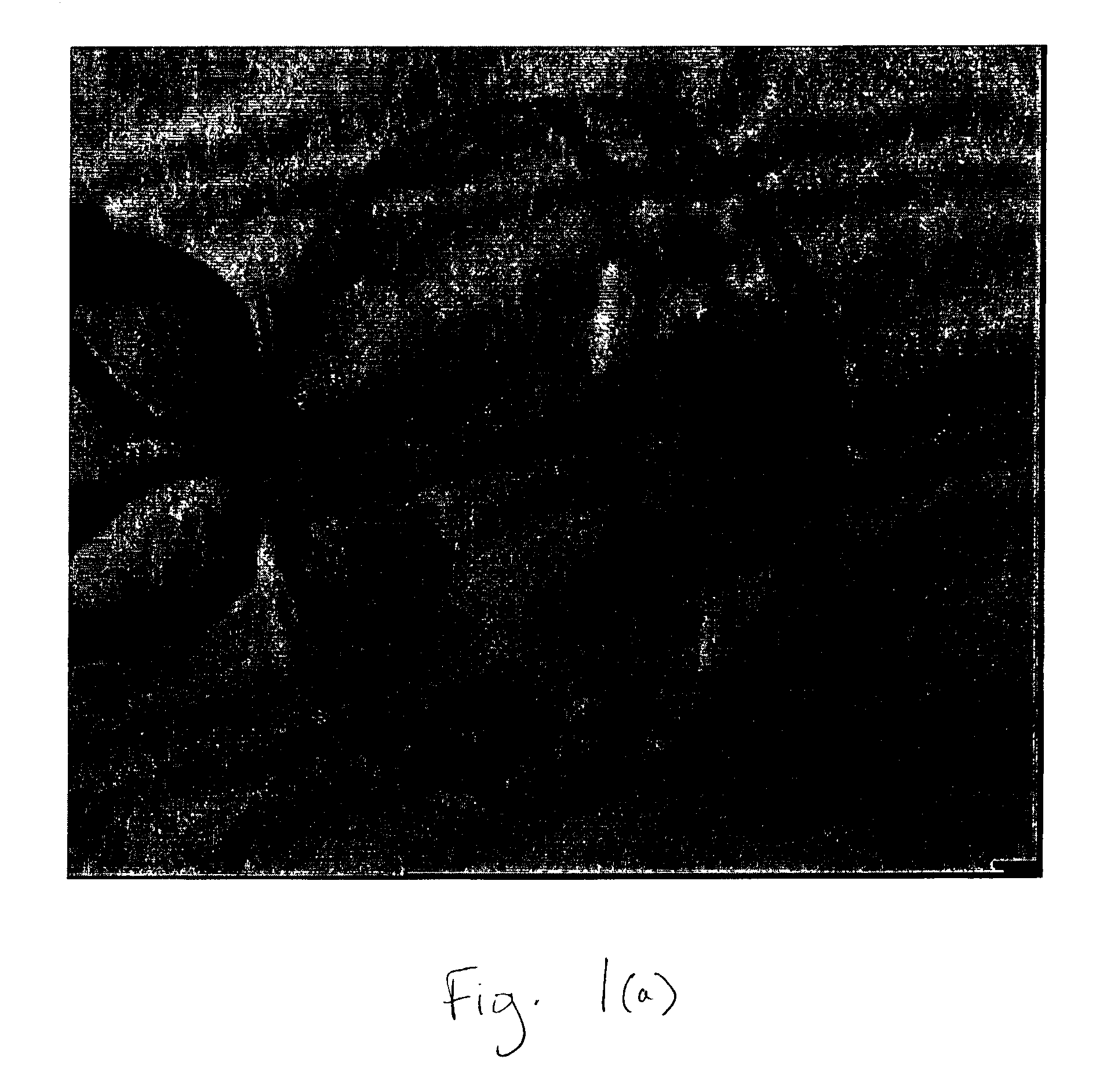
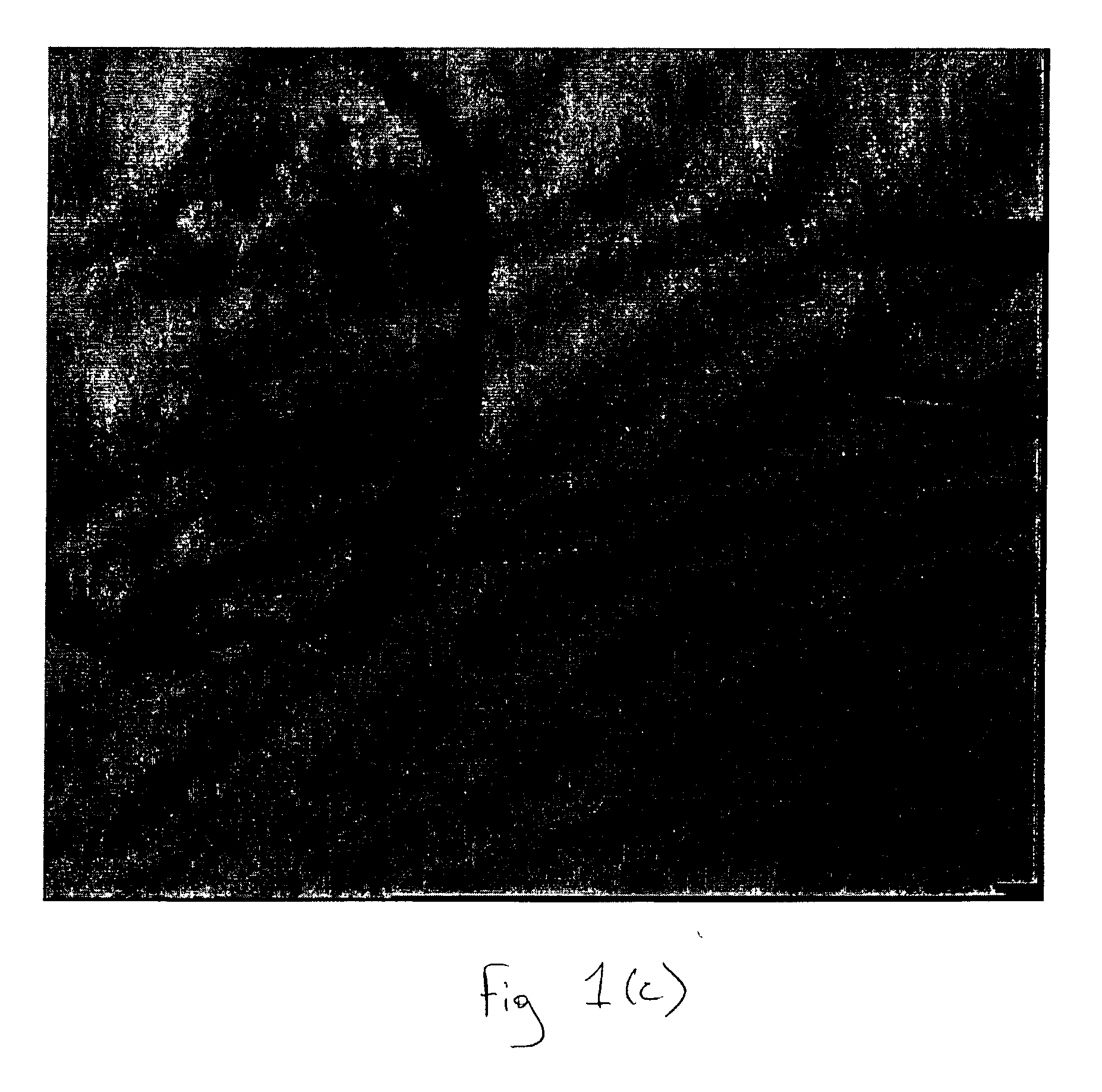
Isolation of Inner Cell Mass for the Establishment of Human Embryonic Stem Cell (hESC) Lines
a technology of embryonic stem cells and inner cells, which is applied in the field of isolate the inner cell mass for the establishment of human embryonic stem cells (hesc) lines, can solve the problems of low percentage of blastocysts hatching spontaneously in humans, the chance of isolating trophectoderm cells, and the inability to isolate the complete inner cell mass
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0073] The following examples are intended to illustrate the invention but do not limit the scope thereof.
example i
[0074] Total of 24 blastocyst stage human embryos were used for the isolation of inner cell mass. Embryos were washed several times in blastocyst culture medium (ISM-2 medium, Medicult, Denmark). Individual blastocyst was then placed in the 50 μl drop of Ca++ / Mg++ free embryo biopsy medium(EB 10 medium, Scadinavian). Micro drops were covered with mineral oil. Micromanipulator was set up. A glass holding pipette with outer diameter 75 μm and inner diameter 15 μm was used to secure the embryo. Biopsy pipette with an outer diameter of roughly 49 μm and inner diameter 35 μm was used for aspiration of inner cell mass. A pilot laser was used to target the main ablation laser. Embryo was immobilized on to the holding pipette in such a way that inner cell mass remained at 3 O'clock position and the zona pellucida and trophectoderm close to inner cell mass positioned to the aiming spot. The hole was induced by a local thermo-dissolution of the zona. Trophectoderm cells were ablated by giving...
example 2
[0076] The objective was to determine efficiency of isolation of inner cell mass with conventional method I.e. immunosurgery and compared with newly invented laser ablated method.
[0077] 21 blastocyst stage human embryo were used for isolation of inner cell mass. Embryos were washed several times with blastocyst culture medium (ISM-2 medium) and followed by ES medium. Individual blastocyst stage embryo was then placed in 50 μl micodrops of 1:50 anti-human antibody (Sigma) for 30 minutes at 37 o C and 5% CO2 in air. Blastocyst stage embryos were then washed four times after incubation with ES medium. Blastocysts were then again placed in 50 μl of microdrops of guinea pig complement at the concentration of 1:10 for 10 minutes at 37 oC and 5% CO2 in air. After incubation blastocyst stage embryos were washed several times in ES medium using fine bore glass pipette in order to remove trophectoderm. Isolated inner cell mass was then washed with ES medium and cultured in 96 well plate in t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com