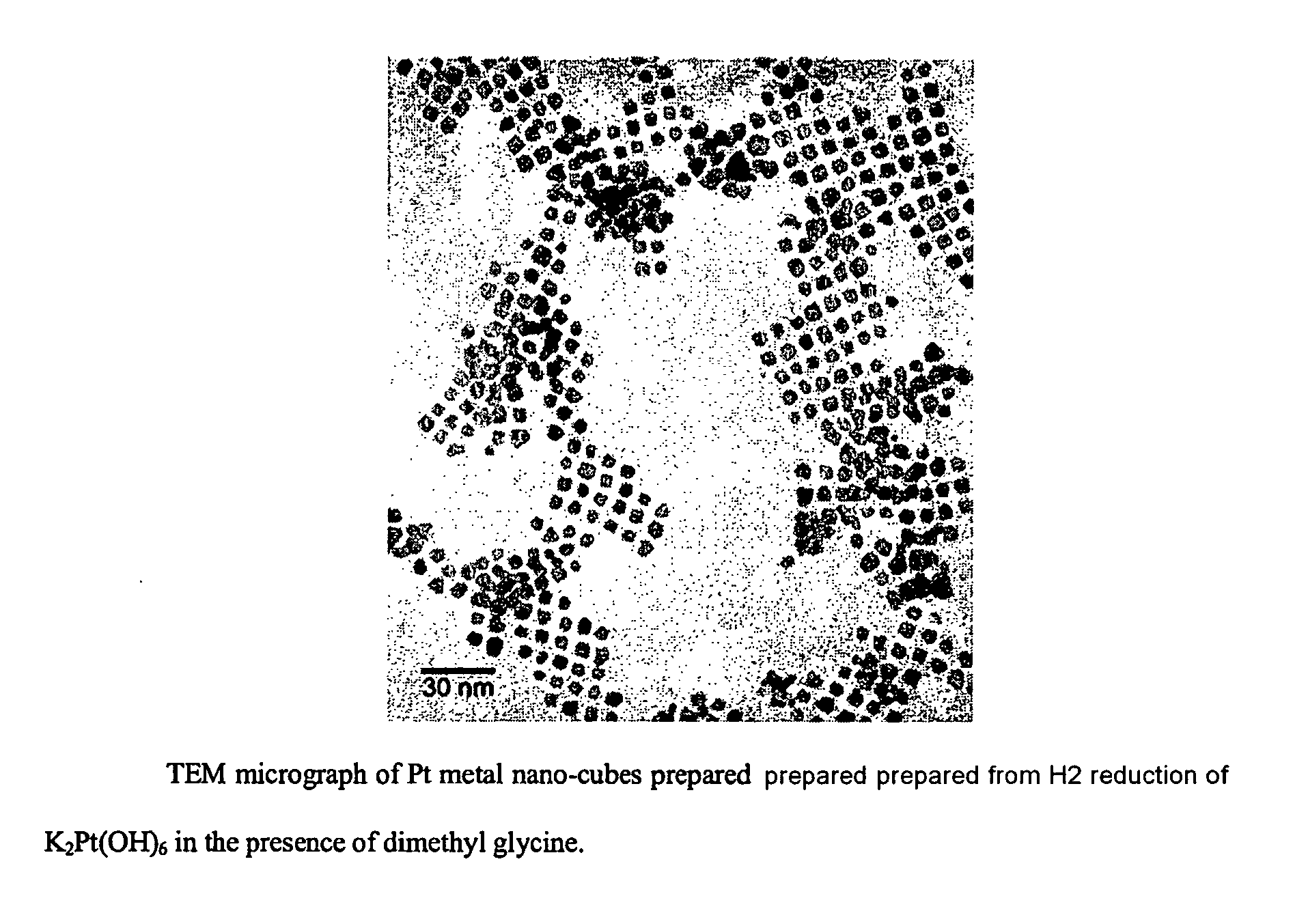
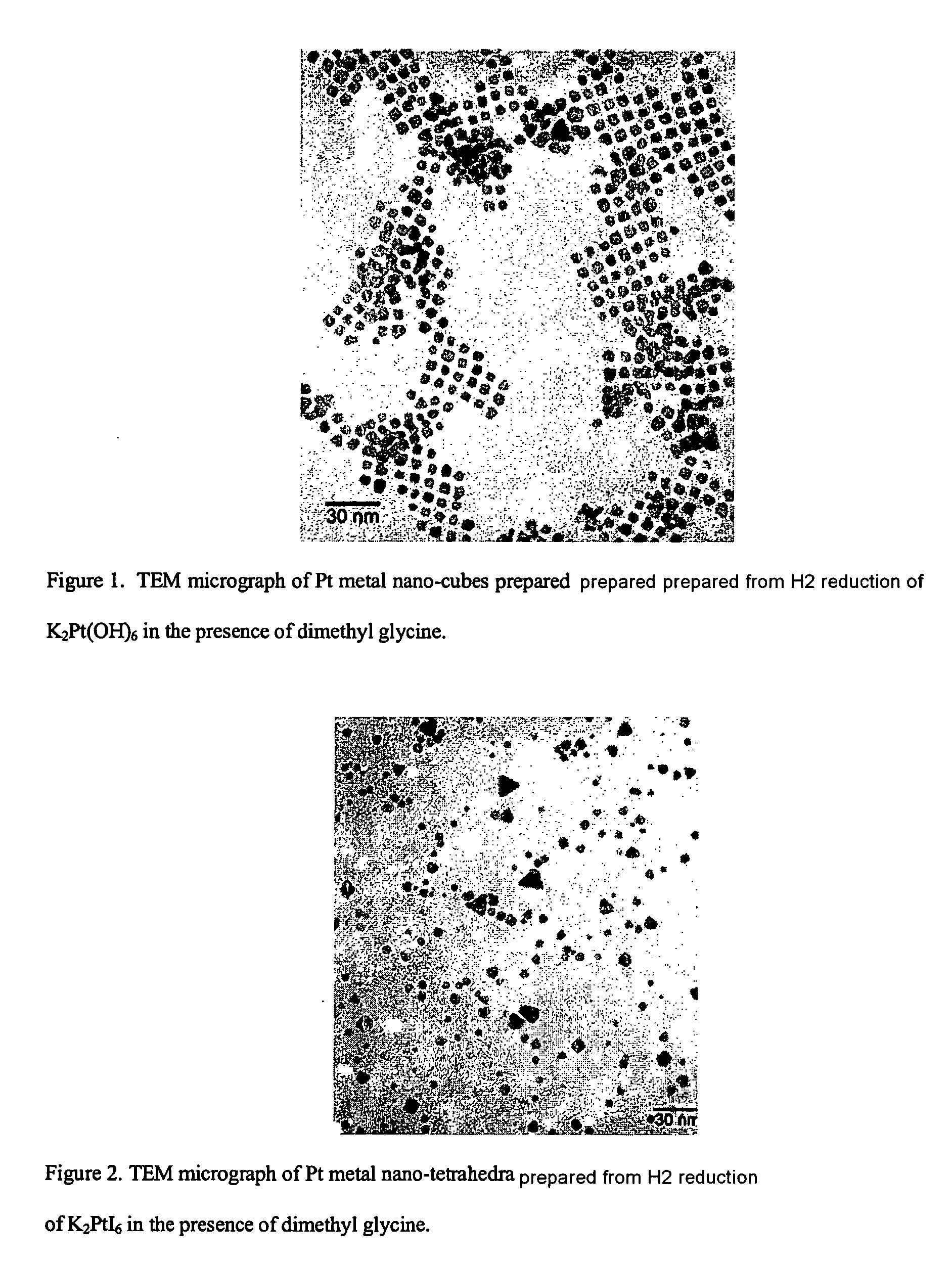
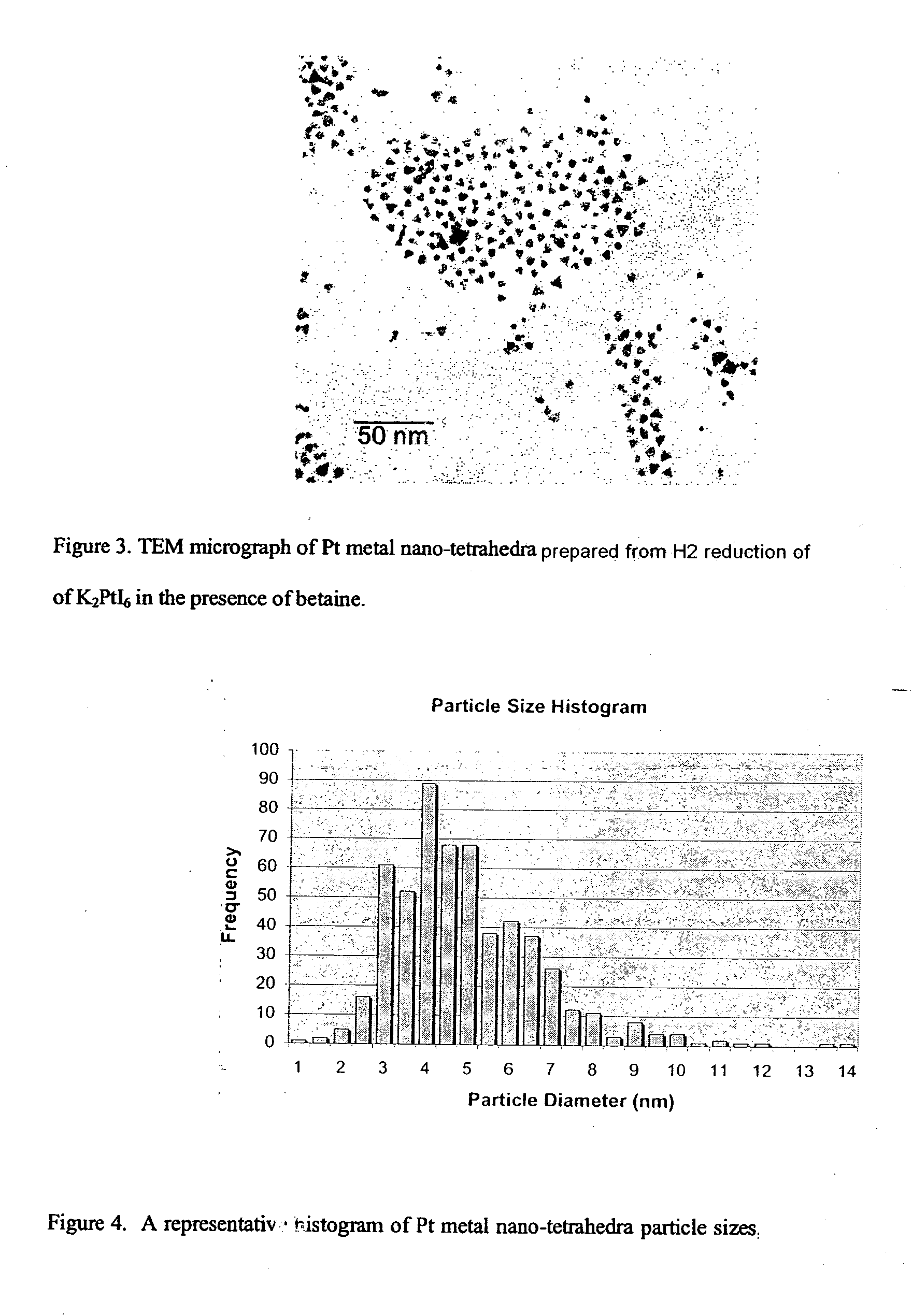
Synthesis of shape-specific transition metal nanoparticles
a transition metal and nanoparticle technology, applied in the field of colloidal particle production, can solve the problems of dramatic (5-to-20-fold) enhancement of fuel cell performance, and the prior art method is applicable only for very small-scale production of nanoparticles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0031] Potassium hexaiodoplatinate (IV) was purchased from Alfa Aesar (Ward Hill, Mass.), N,N-dimethylglycine was purchased from Sigma-Aldrich Co. (St. Louis, Mo.), potassium hydroxide was purchased from EMD Chemicals, Inc. (Gibbstown, N.J.), and distilled water was purchased from Fisher Scientific International, Inc. (Hampton, N.H.).
[0032] The water used in this experiment was degassed by bubbling argon through it for about 15 minutes. A 100 mL aqueous solution at pH 10 was then prepared that was 0.1 mM K2PtI6 and 0.6 mM N,N-dimethylglycine. This solution was sealed in a 250 mL round-bottom flask with a rubber septum and hydrogen gas was then bubbled through it for several minutes. Completion of the reaction was indicated by the solution attaining a golden hue, at which point it had become a colloid containing tetrahedral platinum nanocrystals.
[0033] The shapes of the resulting platinum nanocrystals were verified by transmission electron microscopy (TEM). Their composition as pla...
example 2
[0034] The chemical reagents used in this example are the same as in Example 1 except that N,N-dimethylglycine had been replaced by glycine purchased from Fisher Scientific International, Inc. (Hampton, N.H.).
[0035] The water used in this experiment was degassed by bubbling argon through it for about 15 minutes. A 100 mL aqueous solution at pH 10 was then prepared that was 0.1 mM K2PtI6 and 0.6 mM glycine. This solution was sealed in a 250 mL round-bottom flask with a rubber septum and hydrogen gas was then bubbled through it for several minutes. Completion of the reaction was indicated by the solution attaining a golden hue, at which point it had become a colloid containing tetrahedral platinum nanocrystals.
[0036] The shapes of the resulting platinum nanocrystals were verified by transmission electron microscopy (TEM). Their composition as platinum was verified by energy dispersive spectroscopy (EDS) while the sample was in the TEM instrument. Samples were prepared for TEM analys...
example 3
[0037] The chemical reagents used in this example are the same as in Example 1 except that N,N-dimethylglycine had been replaced by N-methylglycine (sarcosine) purchased from Acros Organics (Geel, Belgium).
[0038] The water used in this experiment was degassed by bubbling argon through it for about 15 minutes. A 100 mL aqueous solution at pH 10 was then prepared that was 0.1 mM K2PtI6 and 0.6 mM N-methylglycine (sarcosine). This solution was sealed in a 250 mL round-bottom flask with a rubber septum and hydrogen gas was then bubbled through it for several minutes. Completion of the reaction was indicated by the solution attaining a golden hue, at which point it had become a colloid containing tetrahedral platinum nanocrystals.
[0039] The shapes of the resulting platinum nanocrystals were verified by transmission electron microscopy (TEM). Their composition as platinum was verified by energy dispersive spectroscopy (EDS) while the sample was in the TEM instrument. Samples were prepar...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Isoelectric point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com