Fabrication of vascularized tissue using microfabricated two-dimensional molds
a two-dimensional mold and vascularization technology, applied in the field of organ transplantation and reconstructive surgery, can solve the problems of limiting the size of newly formed tissue, countless patients suffer, and critical organ shortage,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Micromachining of Template to Tissue Engineer Branched Vascularized Channels for Liver Fabrication.
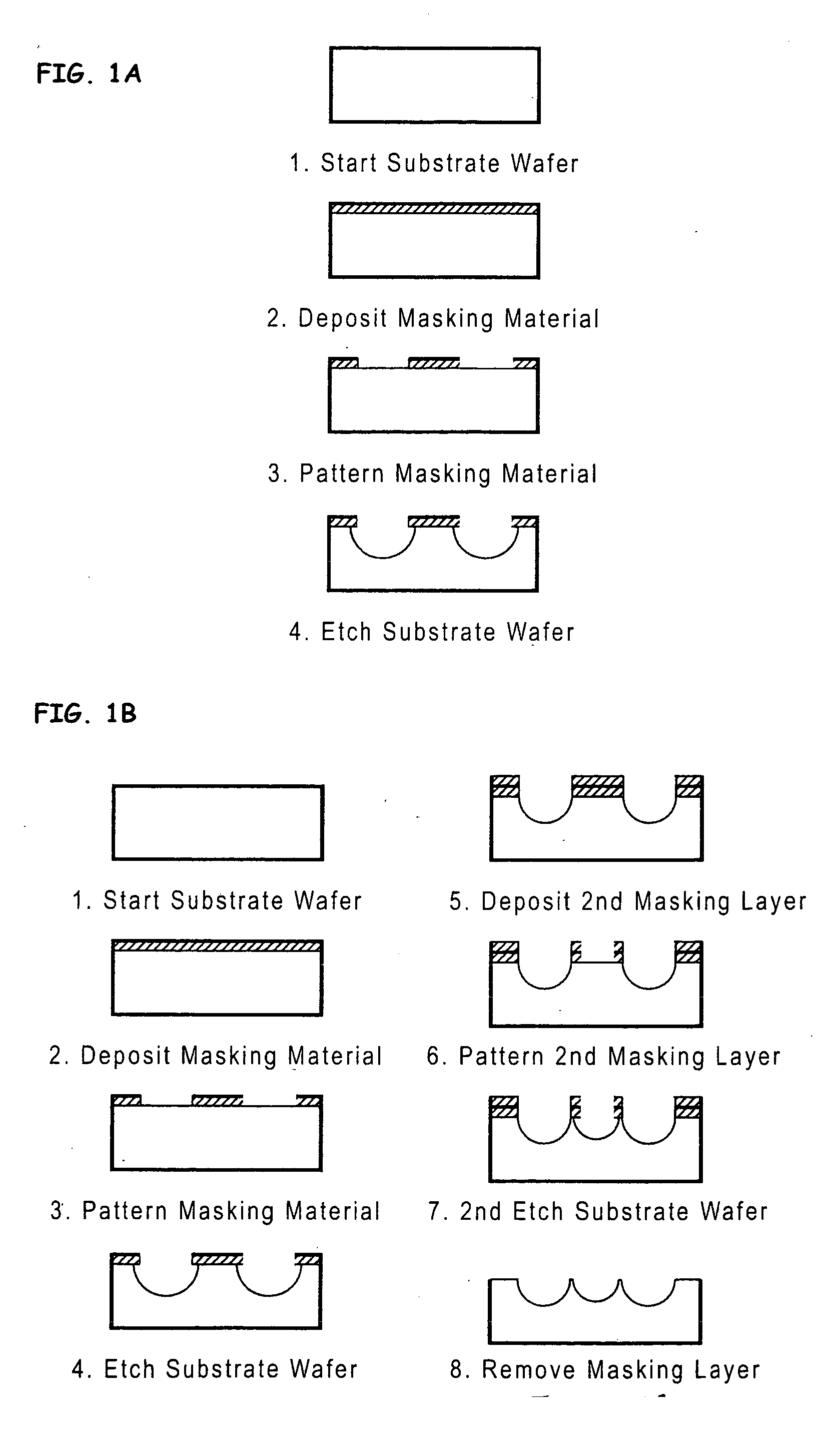
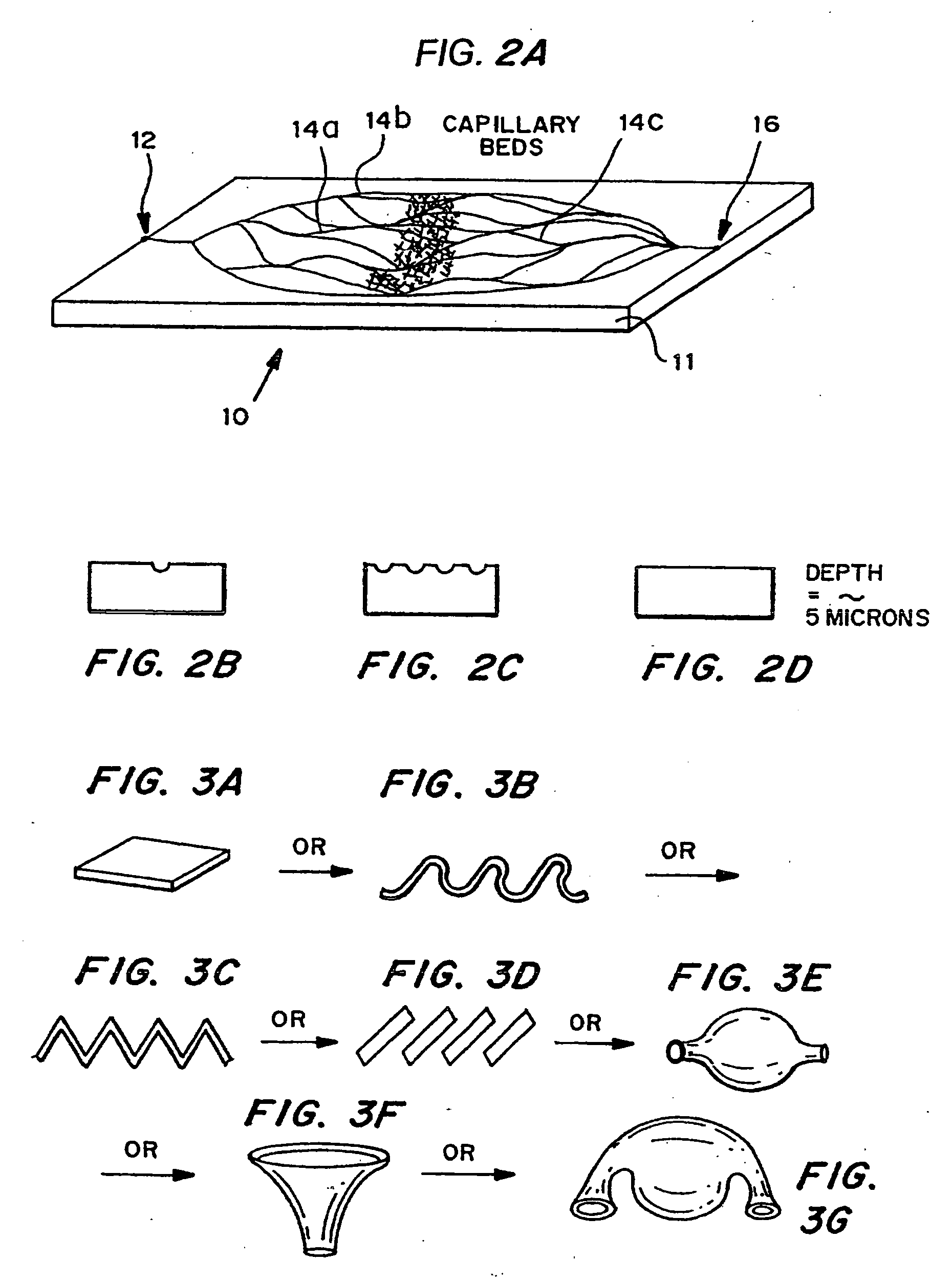
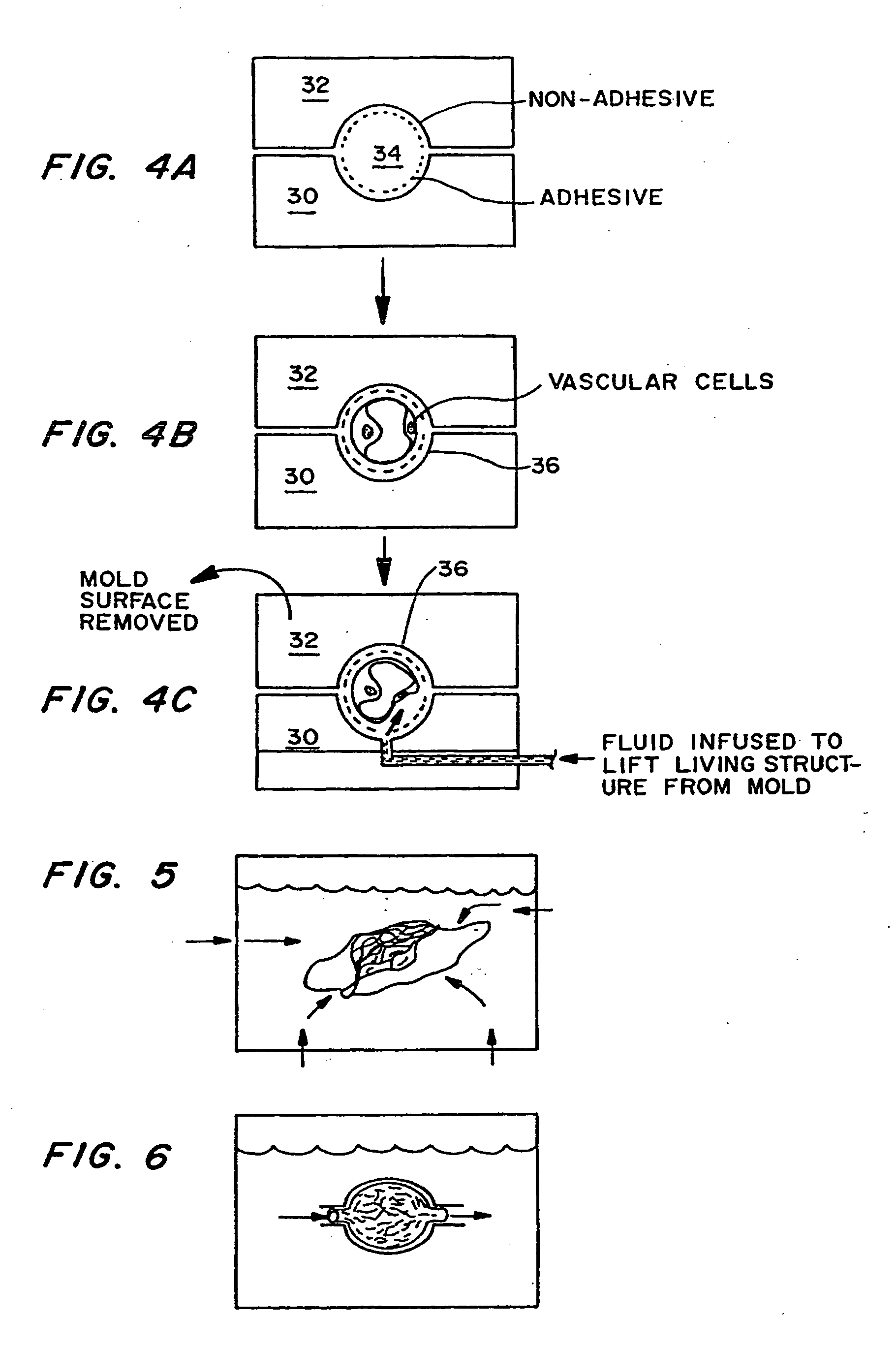
[0086] Micromachining technologies were used on silicon and pyrex surfaces to generate complete vascular systems that may be integrated with engineered tissue before implantation. Trench patterns reminiscent of branched architecture of vascular and capillary networks were etched using standard photolithographic techniques onto silicon and pyrex surfaces to serve as templates. Hepatocytes and endothelial cells were cultured and subsequently lifted as single-cell monolayers from these two dimensional molds. Both cell types were viable and proliferative on these surfaces. In addition, hepatocytes maintained albumin production. The lifted monolayers were then folded into compact three-dimensional tissues. The goal is to lift these branched vascular networks from two dimensional templates so that they can be combined with layers of parenchymal tissue, such as hepatocytes, to form three di...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com