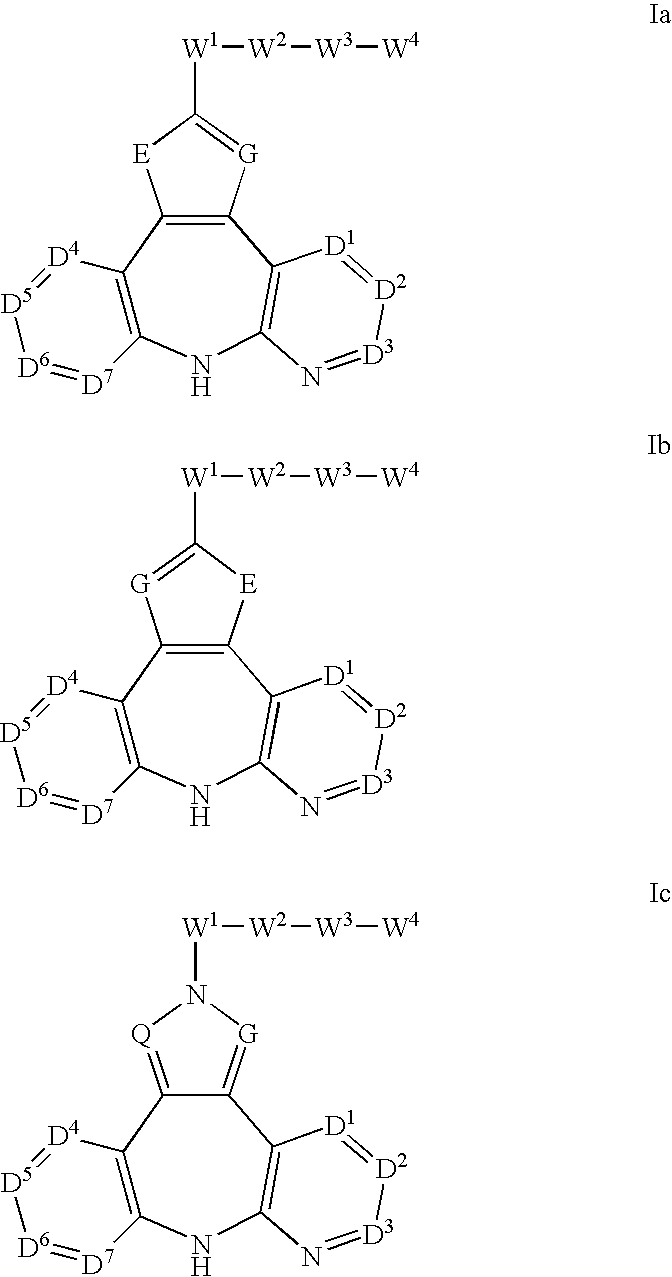
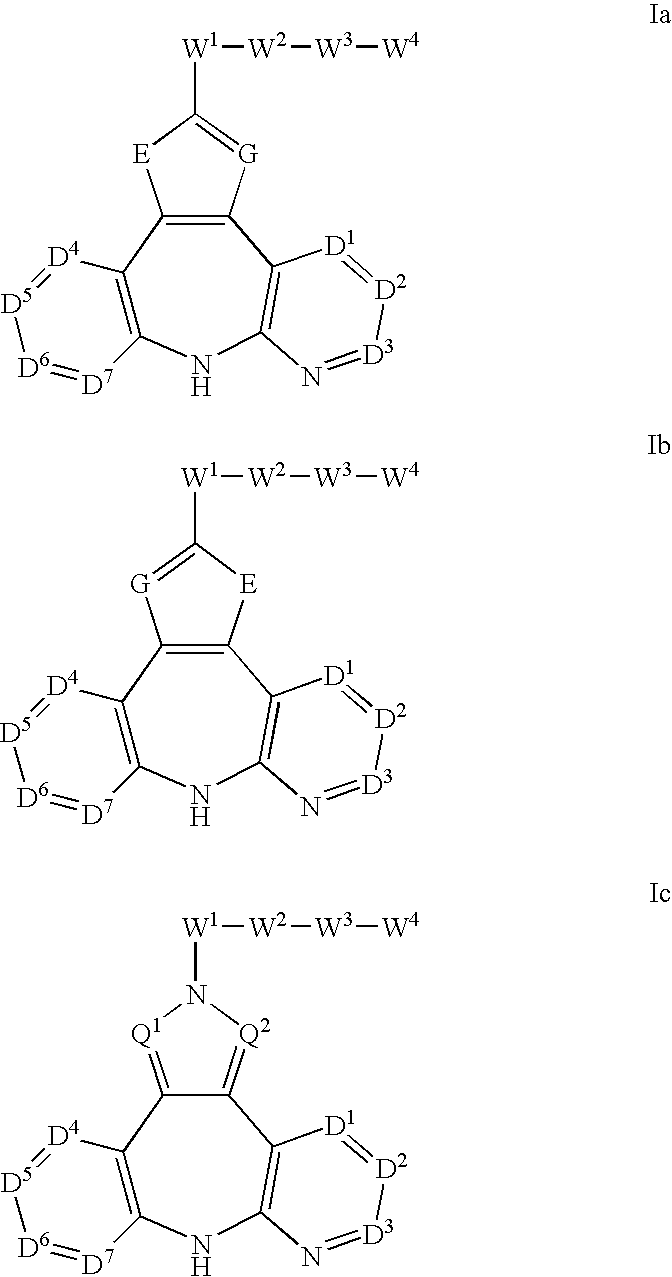
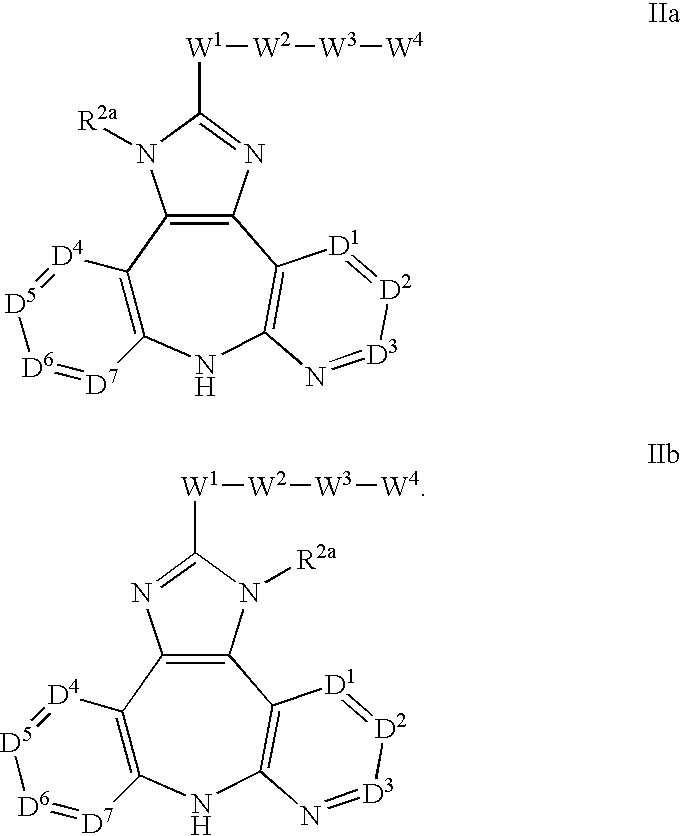
Azepine inhibitors of Janus kinases
a technology of janus kinase and azepine, which is applied in the field of compounding, can solve the problems of adversely affecting patient health, poor prognosis, and elevated levels of circulatin cytokines, and achieves the effects of improving patient health, reducing the number of patients, and improving the quality of li
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation of example 1
Intermediate A-1: 2-fluoro-3-iodopyridine
[0206] To a 1 neck-round flask N,N-diisopropylamine (20 mL, 0.14 mol) was dissolved in tetrahydrofuran (50 mL) and was cooled to −78° C. To the resulting mixture, 1.6 M of n-butyllithium in hexanes (86 mL, 0.14 mol) was added and the reaction was stirred at −78° C. for 10 minutes and then warmed to 0° C. and stirred for 15 minutes. 2-fluoropyridine (10 mL, 0.1 mol) was dissolved in 50 mL anhydrous tetrahydrofuran and the resulting mixture was cooled at −78° C. The LDA solution from the first flask was then added. The reaction was stirred at −78° C. for 1.5 hours and then iodine (32 g, 0.13 mol) was added. The mixture was stirred at −78° C. for and additional 1 hour and was then quenched with NH4Cl (aq). The reaction was extracted with EtOAc, washed with brine, dried over MgSO4, and concentrated under vacuum. The residue was purified by chromatography on silica gel (10% EtOAc 90% hexanes) to give 16.3 g of the desired product. 1H NMR (400 MHz...
preparation of example 25
Intermediate B-1: 4-[(vinyloxy)methyl]cyclohexylmethyl methanesulfonate
[0214] 4-[(Vinyloxy)methyl]cyclohexylmethanol (2.0 g, 0.010 mol) was dissolved in chloroform (20 mL), cooled to 0° C., and then methanesulfonyl chloride (0.85 mL, 0.011 mol) and triethylamine (1.5 mL, 0.011 mol) were added. The reaction was stirred at 0° C. for 2 hours. The reaction was extracted with ethyl acetate, and the extracts were washed with water, brine, and then dried over MgSO4 and concentrated. The product was purified via column chromatography on silica gel (40% ethyl acetate, 60% hexanes), to give 1.53 g of the desired product as a 3:1 mixture of the two diastereomers. 1H NMR (400 MHz, CDCl3): δ 6.36-6.41 (m, 1H), 4.04-4.10 (m, 2H), 3.94-3.96 (m, 2H), 3.39-3.43 (m, 2H), 2.92 (s, 3H), 0.90-1.80 (m, 10H) (major diastereomer).
Intermediate B-2: 4-[(vinyloxy)methyl]cyclohexylacetonitrile
[0215] 4-[(Vinyloxy)methyl]cyclohexylmethyl methanesulfonate (1.43 g, 0.00576 mol) was dissolved in dimethyl sulfoxi...
preparation of example 41
Intermediate C-1: 2-chloro-N,N-diethylnicotinamide
[0220] Into a 1-neck round-bottom flask under an atmosphere of nitrogen was dissolved 2-chloronicotinoyl chloride (2.12 g, 0.0120 mol) in methylene chloride (25 mL) and the reaction cooled to 0° C. N-ethylethanamine (2.7 mL, 0.026 mol) was added to the mixture and stirred at 0° C. for 2 hours and then at 20° C. for 16 hours. The reaction was extracted with dichloromethane and the organic extracts were washed with water, brine, dried (MgSO4), and concentrated in vacuo. The crude product was used in the next reaction without further purification. 1H NMR (400 MHz, CDCl3): δ 8.42-8.44 (m, 1H), 7.64-7.65 (m, 1H), 7.29-7.63 (m, 1H), 3.75-3.79 (m, 1H), 3.35-3.40 (m, 1H), 3.13-3.22 (m, 2H), 1.82 (t, 3H), 1.088 (t, 3H). MS: [M+1]=213.
Intermediate C-2: N,N-diethyl-2-[(4-methylpyridin-3-yl)amino]nicotinamide
[0221] Into a 1-neck round-bottom flask was added 4-methylpyridin-3-amine (1.02 g, 0.00940 mol) and 2-chloro-N,N-diethylnicotinamide (2....
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com