Porous metal oxide and method of preparing the same
a technology powder, which is applied in the direction of nickel compounds, physical/chemical process catalysts, cell components, etc., can solve the problems of difficult preparation of porous metal oxide powder, and difficult application of nano-materials in the practical application. achieve the effect of easy control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
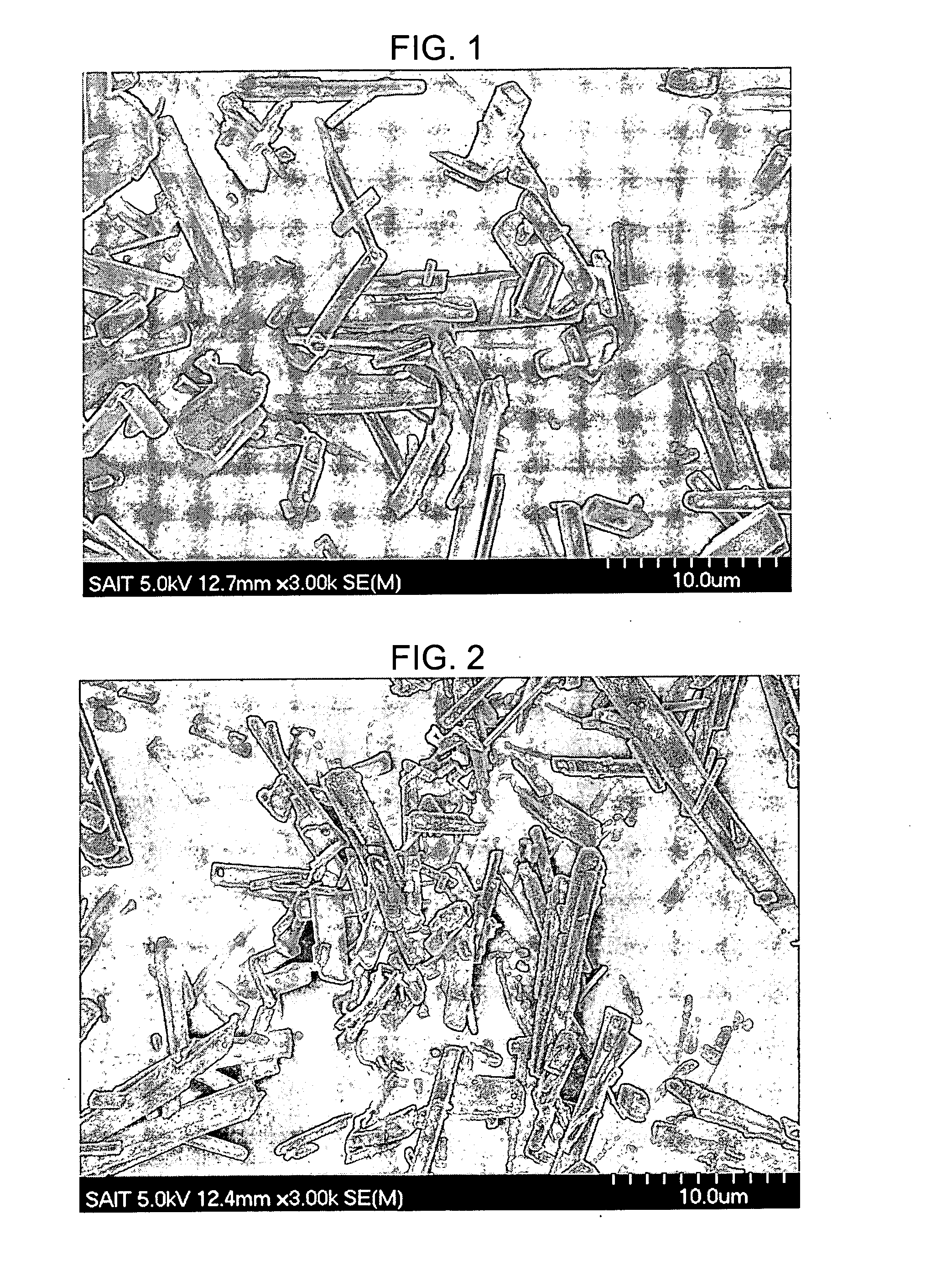
[0054]37.33 g of nickel (II) acetate tetrahydrate and 19.96 g of trimesic acid were added to 500 ml of distilled water and stirred at 55° C. for 2 hours. Powders produced in the solution were removed using a nylon filter, washed with distilled water several times, and then dried in an oven at 80° C. for 12 hours to obtain a crystalline coordination polymer. FIG. 1 is a scanning electron microscope (SEM) image of the crystalline coordination polymer prepared according to this example.
[0055]The obtained crystalline coordination polymer was subjected to heat treatment under an Ar atmosphere at 600° C. for 1 hour to prepare a carbon-nickel composite having the same shape as the untreated crystalline coordination polymer and a reduced volume. FIG. 2 is a SEM image of the obtained carbon-metal composite.
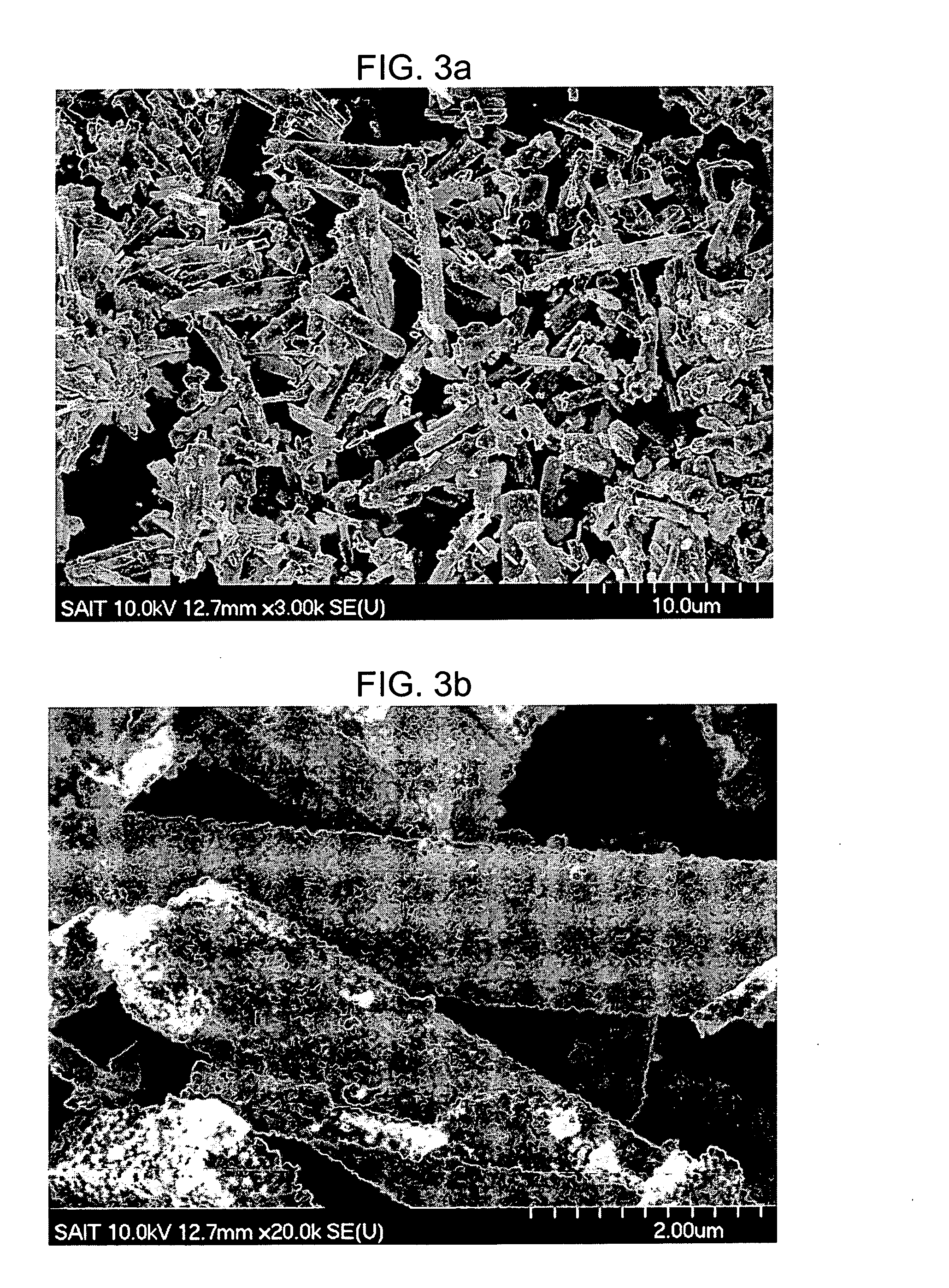
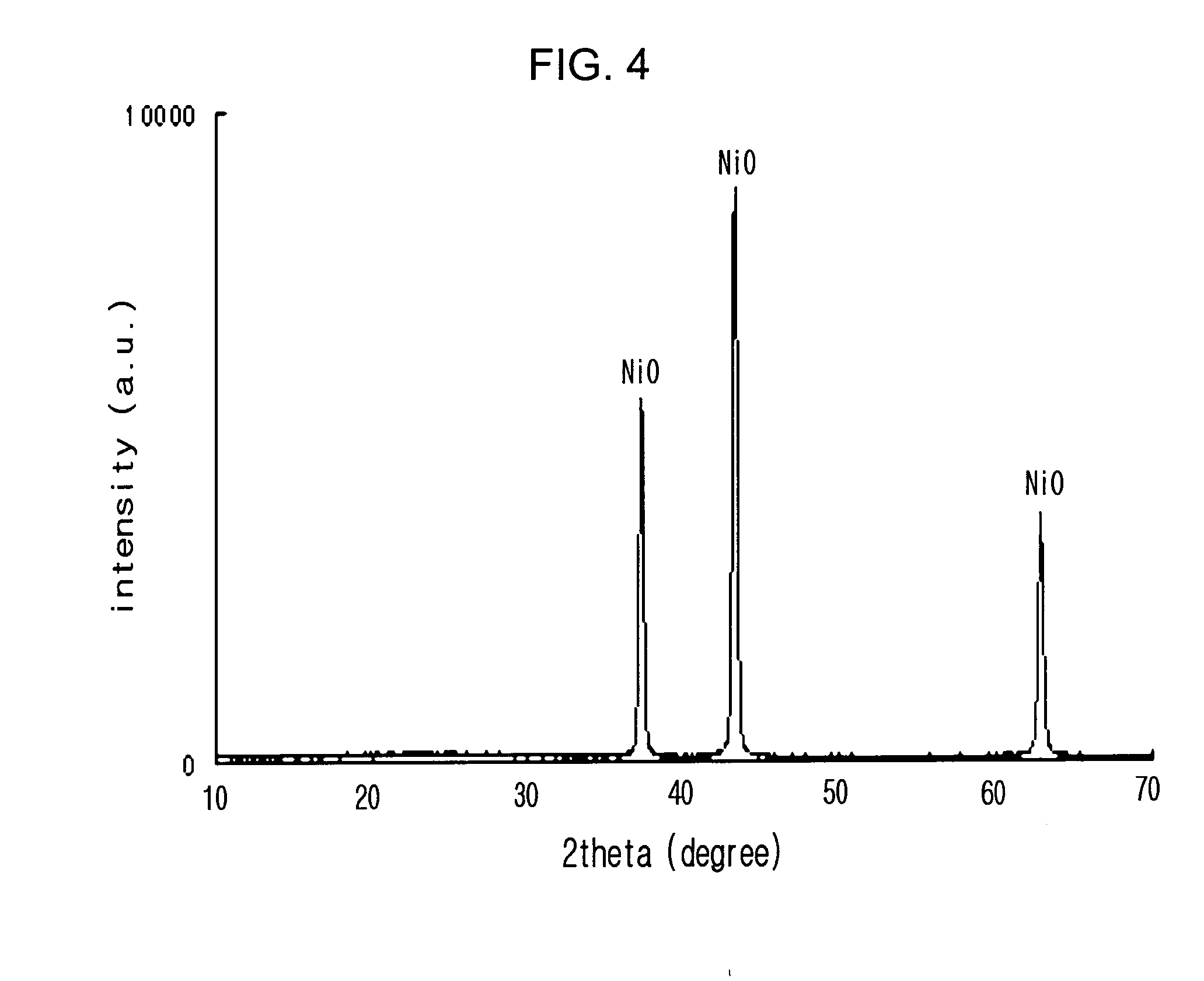
[0056]FIGS. 3A and 3B are SEM images of a porous nickel oxide obtained by heat treating the obtained carbon-nickel composite in air at 700° C. for 1 hour. FIGS. 3A and 3B show that the oxi...
examples 2 through 5
[0057]Synthesis of the coordination polymer and heat treatment were performed as in Example 1, except that the heat treatment temperature for forming the carbon-metal composite was adjusted from 700 to 1000° C. as listed in Table 1 below.
TABLE 1Heat treatment temperature (° C.)First heatSecond heatSamplePrecursortreatment (Argon)treatment (Air)Example 2Nickel(II) trimesate700700Example 3Nickel(II) trimesate800700Example 4Nickel(II) trimesate900700Example 5Nickel(II) trimesate1000700
FIGS. 6A and 6B are SEM images of the porous nickel oxide prepared according to Example 2. FIGS. 7A and 7B are SEM images of the porous nickel oxide prepared according to Example 3. FIGS. 8A and 8B are SEM images of the porous nickel oxide prepared according to Example 4. FIGS. 9A and 9B are SEM images of the porous nickel oxide prepared according to Example 5.
[0058]These results show that the size of the primary particles and the diameters of the pores increase as the temperature increases from a heat tr...
example 6
[0061]14.93 g of nickel (II) acetate tetrahydrate, 3.73 g of cobalt (II) acetate tetrahydrate, and 9.98 g of trimesic acid were added to 500 ml of distilled water and stirred at 55° C. for 2 hours. Powders produced in the solution were removed using a nylon filter, washed with distilled water several times, and then dried in an oven at 80° C. for 12 hours to obtain a needle-shaped coordination polymer crystal.
[0062]The obtained crystalline coordination polymer was subjected to a heat treatment process under an Ar atmosphere at 900° C. for 1 hour to prepare a carbon-(nickel, cobalt) composite, and then subjected to a heat treatment process at 700° C. for 1 hour to prepare a porous Ni0.8Co0.2O material. FIGS. 12A and 12B are SEM images of the prepared porous Ni0.8Co0.2O material.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com