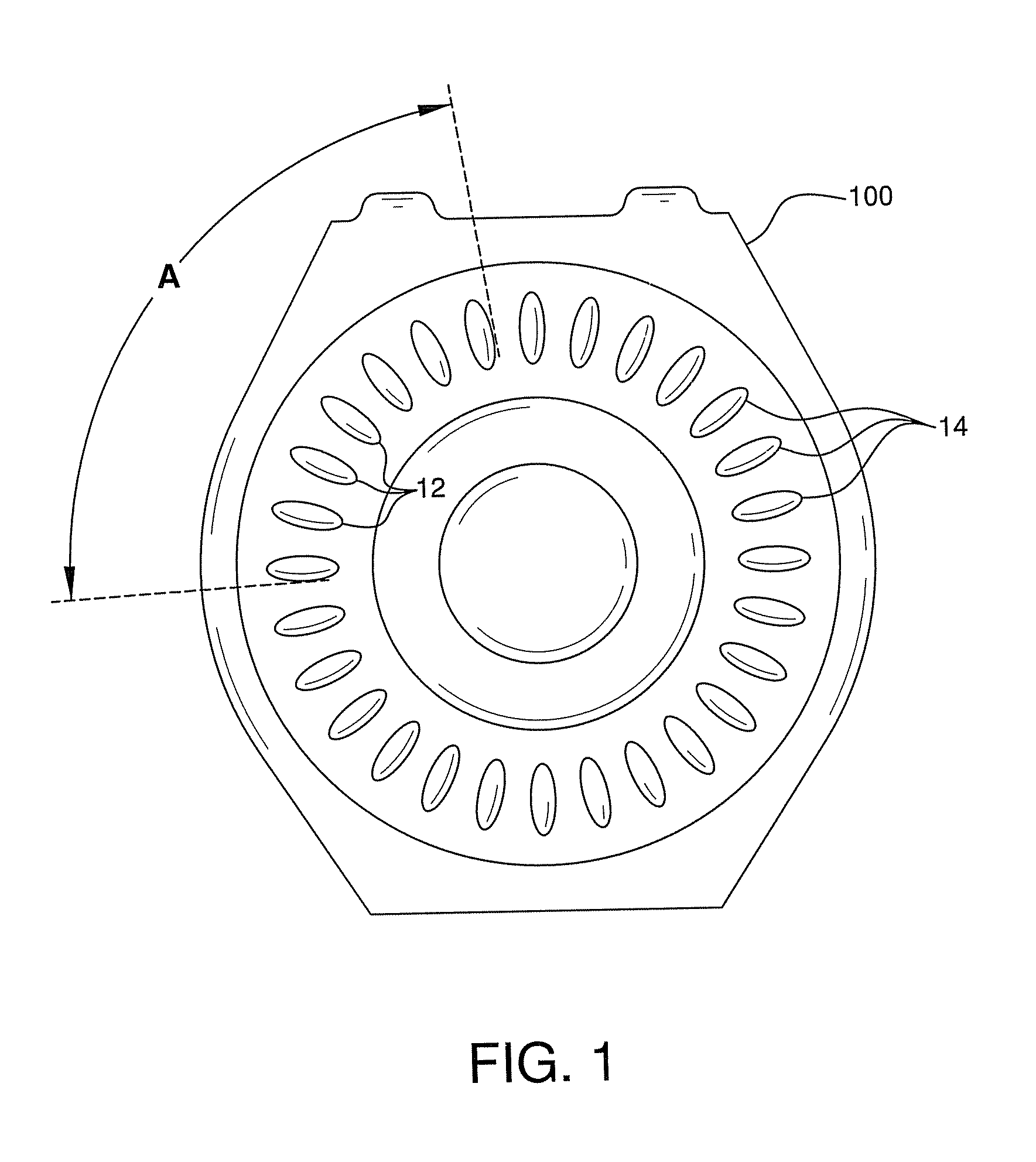
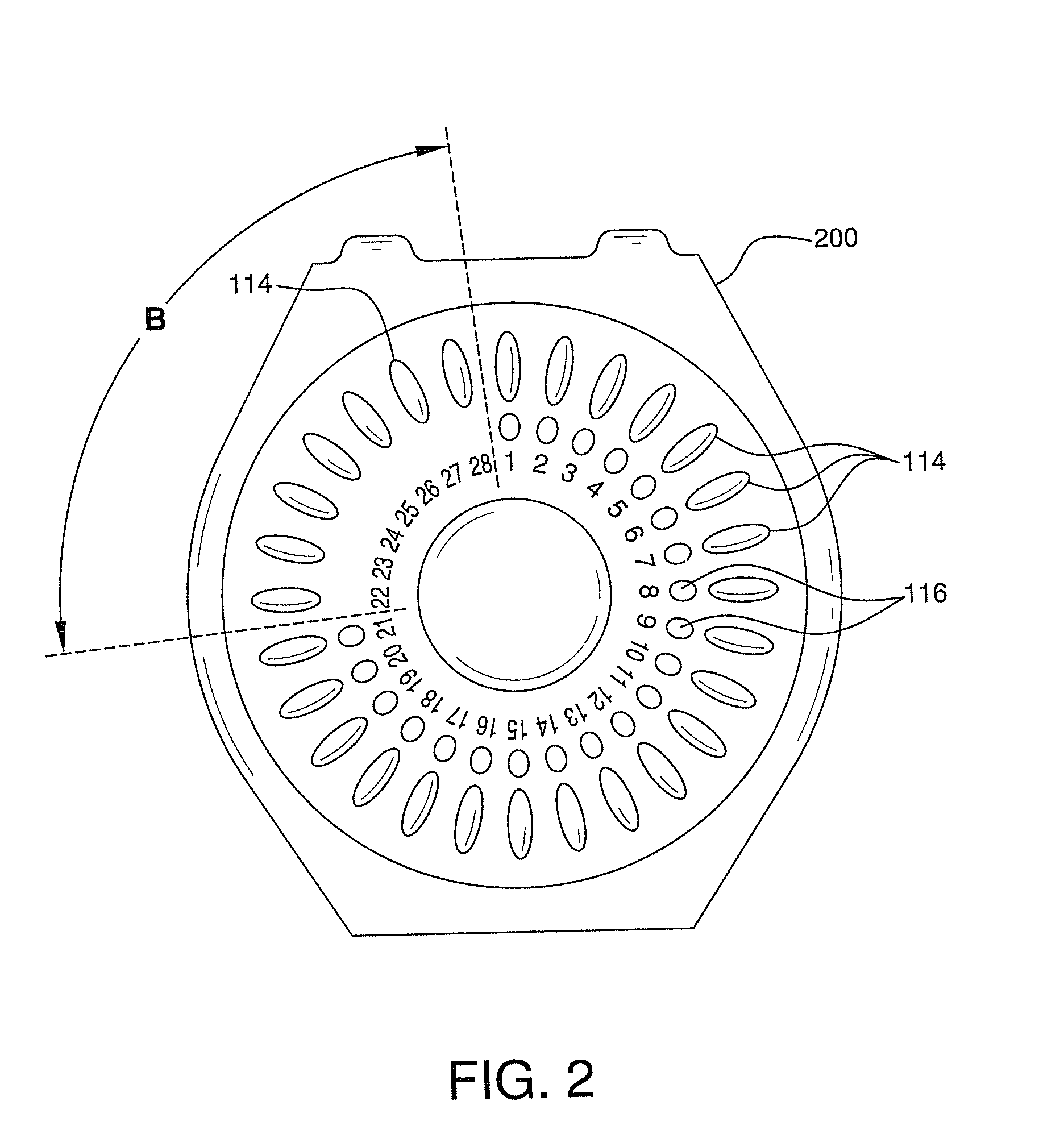
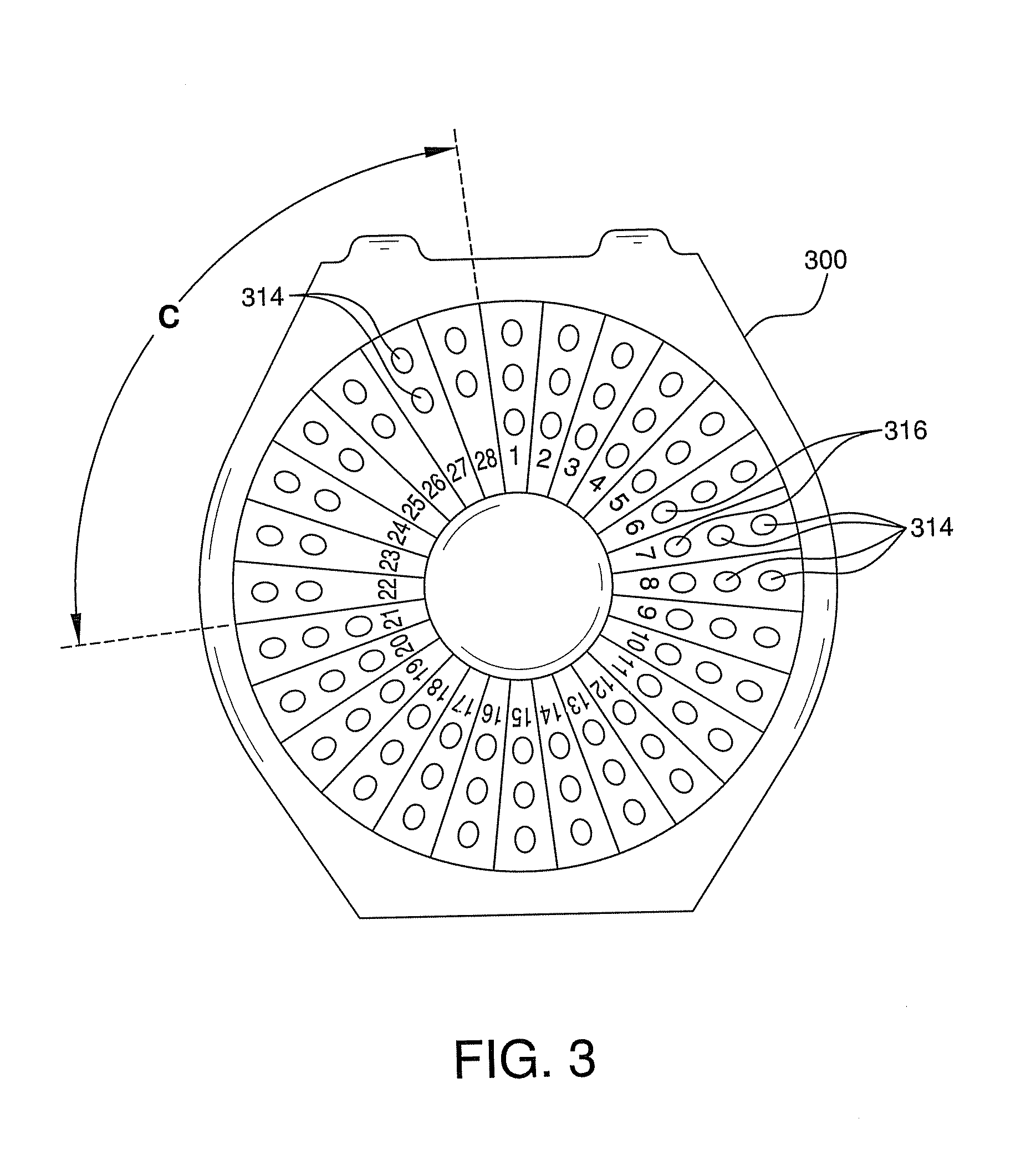
Contraceptive and Acne Medication Combination and Treatment of Acne and Other Diseases with Reduced Side Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0043]Oral isotretinoin (13-cis-retinoic acid or Accutane® or 13-cis vitamin A) is an isomer of all-trans retinoic acid, a metabolite of retinol (vitamin A). The absorption is enhanced when the drug is taken with food. There is no progressive accumulation of the drug in the skin during long term administration. It has a very short half-life compared to etretinate (7-37 hours). It crosses the placenta. It is rapidly eliminated by the liver and kidneys, with the main mechanism of excretion being hepatic clearance. No parent drug is identified in the urine.
[0044]Amongst both synthetic (such as etretinate, acitretin, arotinoids) and natural retinoids (such as all-trans retinoic acid and isotretinoin), only isotretinoin exerts its effect on sebum production and therefore on acne. The most likely mechanism by which isotretinoin leads to clinical improvement in acne is by reducing sebaceous gland size (up to 90%) by decreasing proliferation of basal sebocytes, suppressing sebum production ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
| Weight | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com