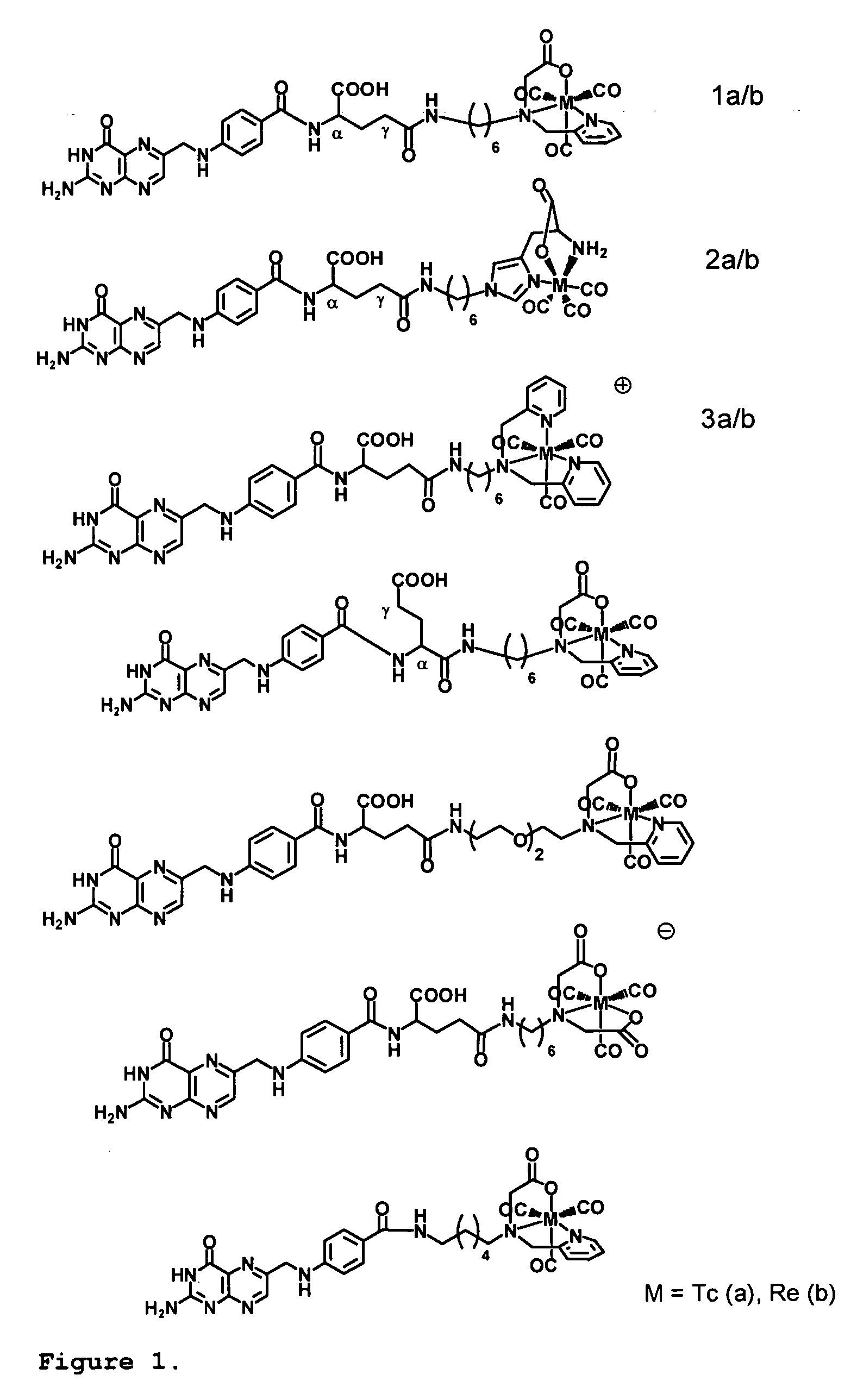
Method for cell-specific targeting
a cell-specific and targeting technology, applied in the field of cell-specific targeting, can solve the problems of limiting the use of currently known folate-mediated targeting methods in various medical applications, the risk of radiation injury in sensitive renal tissue, and the inability to exploit tumour-targeting for its use in radiotherapy, so as to reduce the burden of unwanted doses and precise localisation of malignant cell populations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
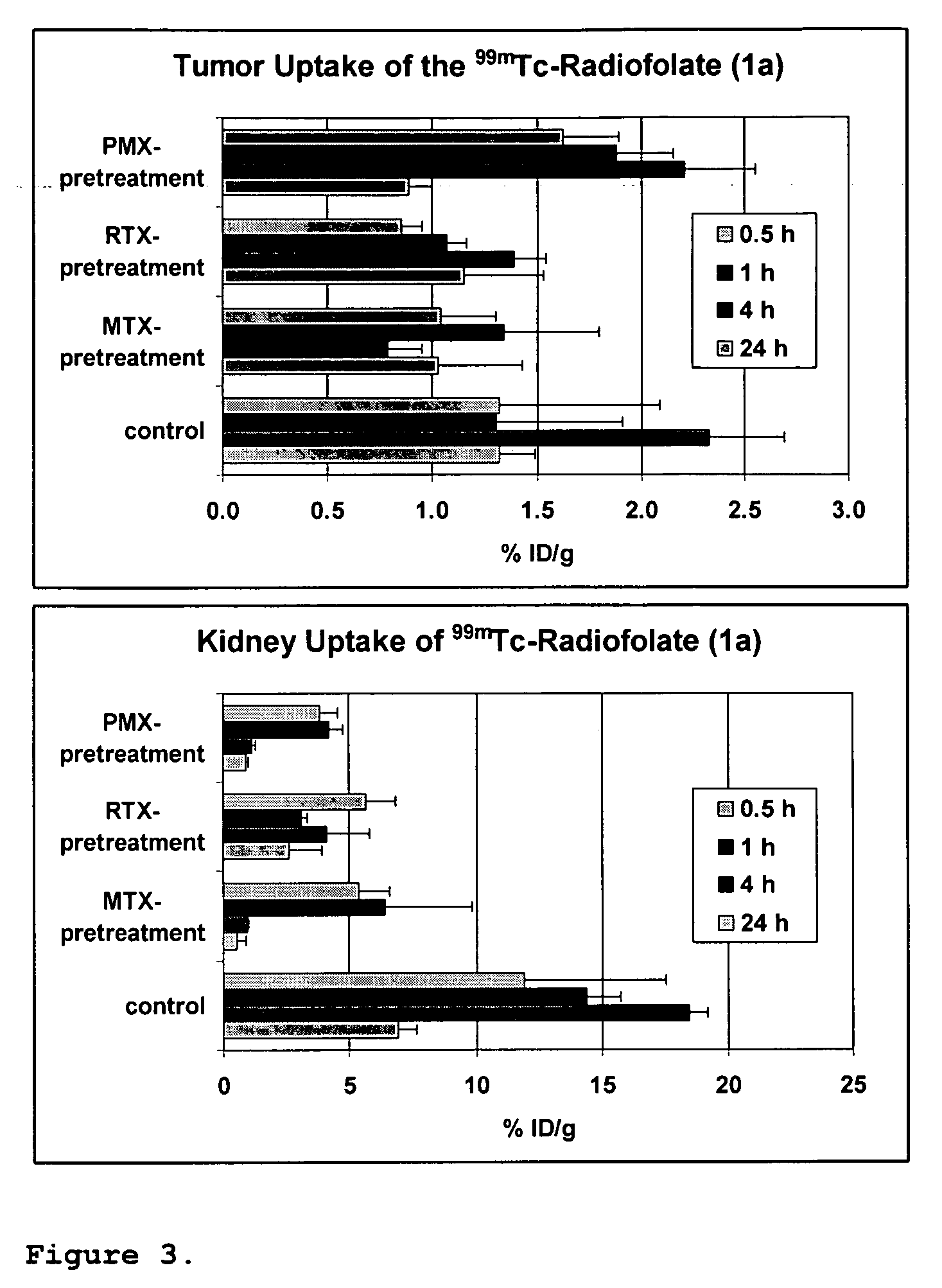
[0063]Materials and Methods. The synthesis of the picolylamine monoacetic acid (PAMA)-folate-conjugate was performed as described in (Müller et al, J. Organomet. Chem. 2004, 689:4712-21). Precursor [99mTc(CO)3(OH2)3]+was prepared using the Isolink™-kit (Mallinckrodt-Tyco, Petten, the Netherlands) (Alberto et al, J. Organomet. Chem. 1995, 492:217-24; Alberto et al, J. Am. Chem. Soc. 2001; 123:3135-36). [Na][99mTcO4] was eluted from a 99Mo / 99mTc-generator (Mallinckrodt-Tyco, Petten, the Netherlands) with a 0.9% saline solution. [Na][188ReO4] was eluted from a 188W / 188Re-generator (Oak Ridge National Laboratories, Oak Ridge, Tenn.). Injection solutions of methotrexate (Amersham Life Science) were prepared with phosphate buffered saline (PBS, pH 7.4) followed by sterile flirtation. The injection solutions of Tomudex® (ralitrexed) and Alimta® (pemetrexed) were prepared according to the package instructions with aqua ad injectabilia or NaCl 0.9% respectively. KB cells (CCL-17) were purcha...
example 2
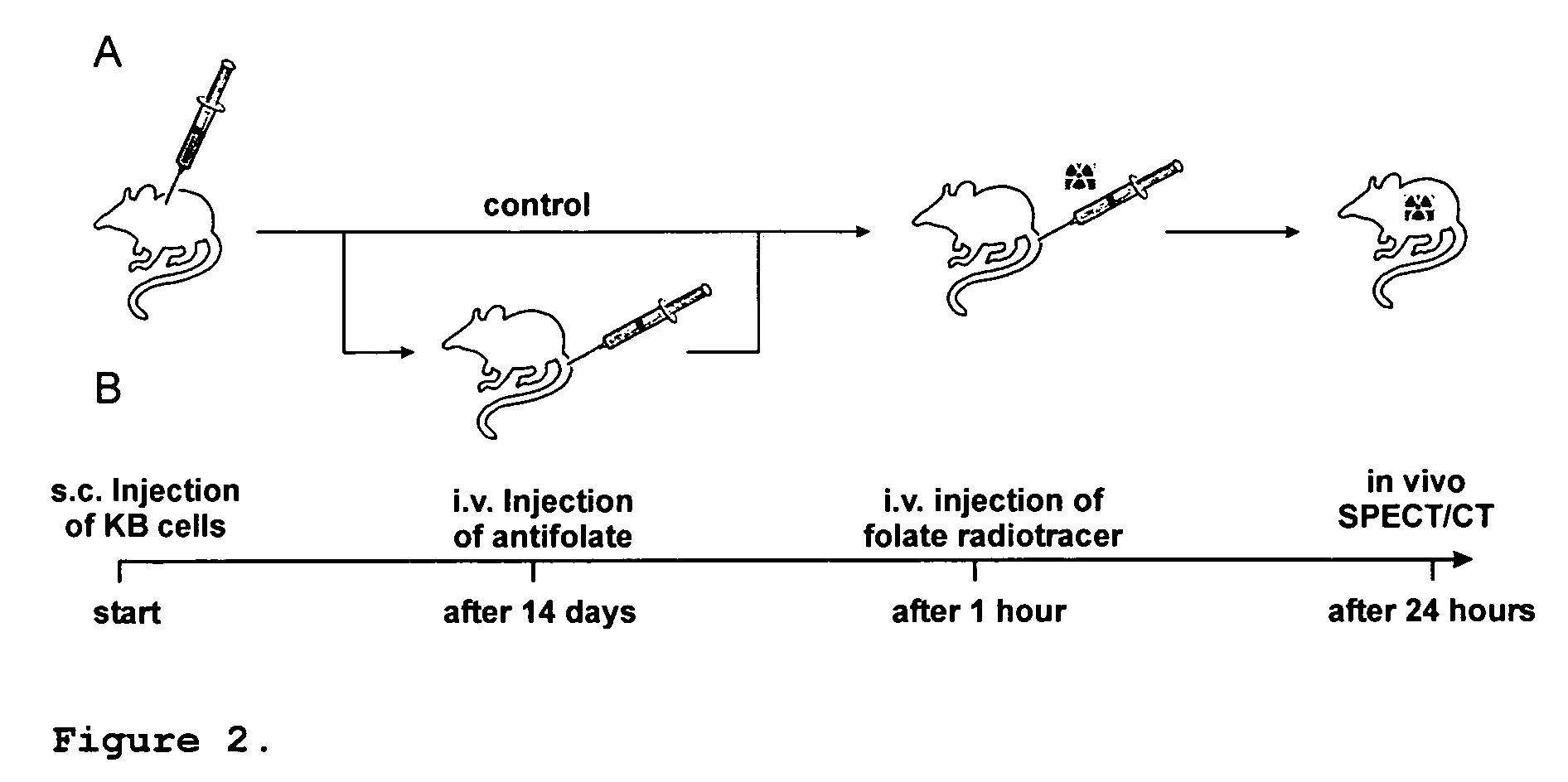
In Vivo Evaluation of Different Folate Radiotracers (2a, 3a) in Combination with MTX and PMX Respectively
[0081]The effect of antifolates with respect to selective protection of kidney tissue is not limited to compound 1a / b but is applicable to structurally different complexes as mentioned above including e.g. complexes 2a and 3a. Biodistribution of the radiotracers 2a and 3a were assessed in mice, with or without pre-treated with PMX or MTX respectively, 1 h previous to the injection of the radiotracer (Table 6).
TABLE 5Biodistribution 4 h p.i. data of radiotracer 2a and 3a in athymic nude mice, bearingKB xenografts with or without PMX or MTX pre-treatment.Organs2a2a + PMXa3a3a + MTXablood0.19 ± 0.180.07 ± 0.020.03 ± 0.010.01 ± 0.00heart0.80 ± 0.600.72 ± 0.390.20 ± 0.090.02 ± 0.00lung0.85 ± 0.080.50 ± 0.190.20 ± 0.020.02 ± 0.00spleen0.30 ± 0.050.19 ± 0.030.08 ± 0.020.01 ± 0.00kidney42.02 ± 7.31 5.05 ± 0.677.11 ± 0.750.78 ± 0.17stomach0.62 ± 0.220.46 ± 0.220.54 ± 0.580.14 ± 0.06intest...
example 3
In Vivo Evaluation of 99mTc(CO)3-Folate (1a) in Combination with MTX Using Different Folate Receptor Positive Tumour Cell Xenografts
[0082]The effect of antifolates with respect to selective protection of kidney tissue is not limited to KB cancer cells but to all cell lines expressing the folate receptor and activated (but not resting) synovial macrophages.
[0083]4-5-week-old female, athymic nude mice (CD1-Foxn1 / nu) were purchased from Charles River Laboratories (Sulzfeld, Germany). Mice were housed under conditions of controlled temperature (26° C.), humidity (68%) and daily light cycle (12 h light / 12 h dark). The animals were fed with a folate-deficient rodent diet (to reduce their serum folate to a level near that of humans) (Mathias et al, J. Nucl. Med. 1996; 37:1003-08). After an acclimation period of 5-7 days, the mice were inoculated subcutaneously with the tumour cell suspension using IGROV-1 and KB-V1 (multiresistant) cells (5×106 cells) into the subcutis of the axilla. Radio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com