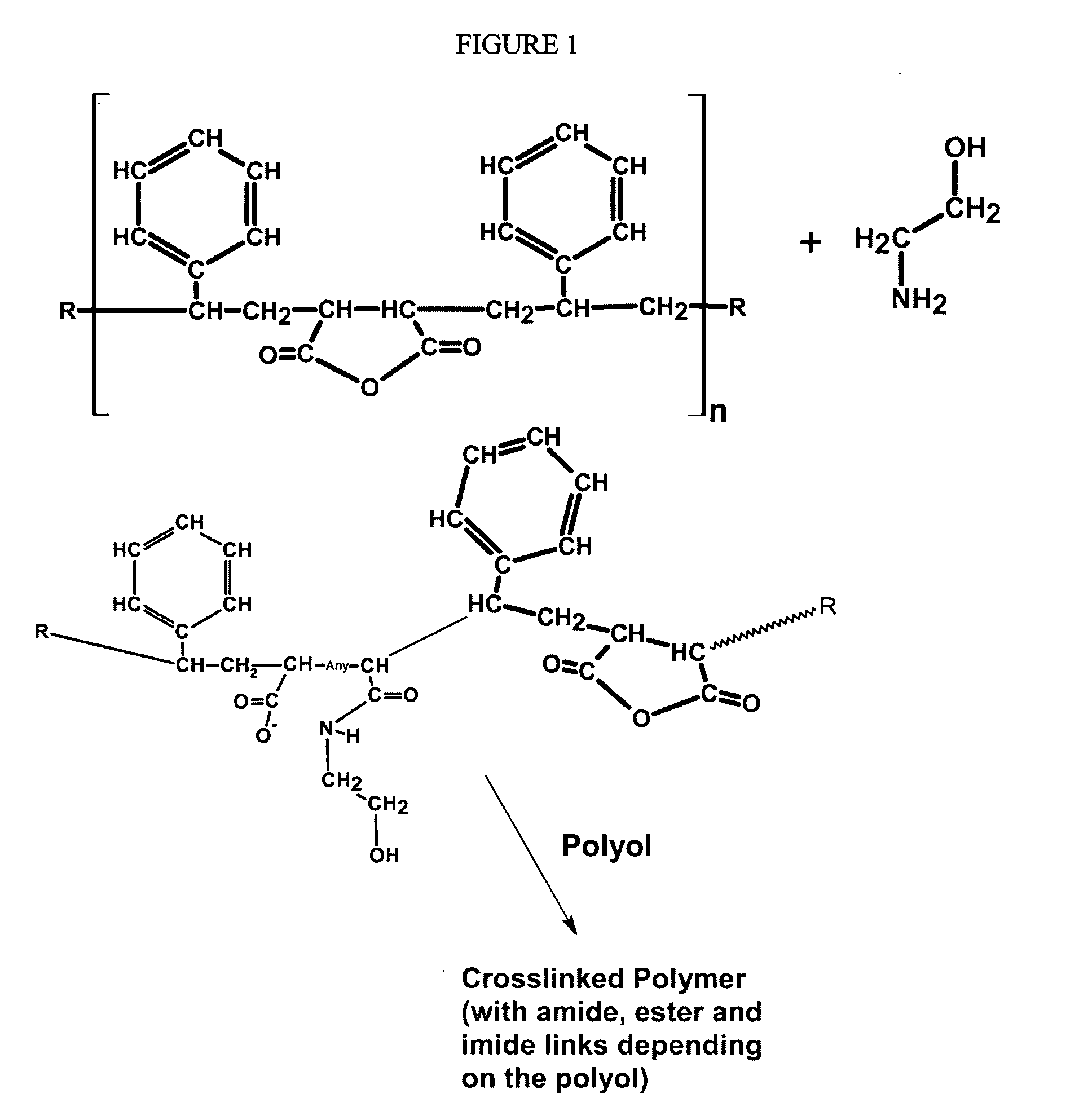
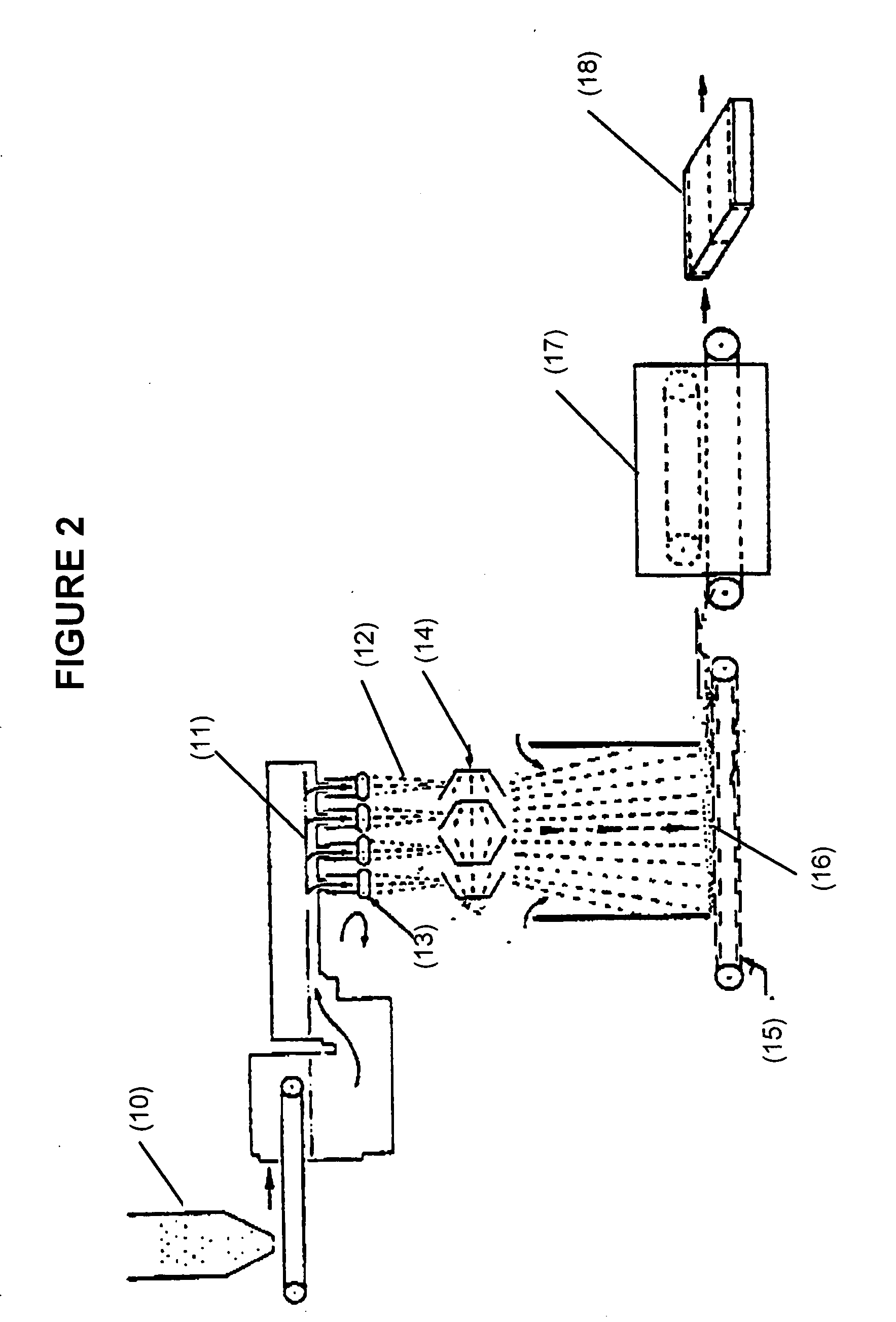
Formaldehyde free binder
a binder composition and formaldehyde-free technology, applied in the field of new formaldehyde-free binder compositions, can solve the problems of heightened risk of incurring accelerated corrosion of process equipment associated with storage, transportation, and application of binder compositions, and the potential for formaldehyde emissions during the preparation of adhesive resins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0082]To a standard resin kettle was added MEA (monoethanolamine—12 g) and DEA (diethanolamine—50 g) and SMA-1000 (from Sartomer) in an amount of 50 g. An exothermic reaction occurred in the mixture (an IR taken of the sample at this point, after about 30 minutes of reaction, showed the presence of a secondary amide). Another 150 g of the SMA-1000 then was added to the reaction mixture, followed by 730 g of water. The reaction mixture was heated to 90° C. for about 2 hours and a clear solution was obtained. The pH of the aqueous resin was 4.5. The pH of the resin was then raised to above 5.5 by the addition of aqueous (28% by weight) ammonia. The resulting resin product, when heated to about 200° C. in an oven showed good cure characteristics. An IR spectrum taken of the cured resin product showed the presence of ester, amid and imide groups.
example 2
[0083]To a standard resin kettle was added 600 g of SMA-1000, 1000 g of water, and 40 g of MEA (monoethanolamine). An exothermic reaction occurred in the mixture. An IR of the sample at this point, after about 30 minutes of reaction time, showed the presence of a secondary amide. At this point, 200 g of TEA then was added. The reaction mixture was heated to 90° C. for about 2 hours after which an additional 600 g of water was added and a clear solution was obtained. The pH of the aqueous resin was at 4.7. The pH of the resin was then raised to above 7.0 by the addition of aqueous ammonia. The IR spectrum of this aqueous resin solution following the neutralization showed the presence of amide and a minor amount of imide and carboxylate salt. The resin product was heated to about 200° C. in an oven and showed good thermosetting characteristics. The IR spectrum of the cured thermoset resin showed the presence of ester, amid and imide groups.
example 3
[0084]To a pressure reactor (Parr) were added 1500 g of water, 150 g of a high molecular weight SMA (Styrene:MA mole ratio of approximately 3:1), 15 .g of monoethanolamine (MEA), 20 g of aqueous ammonia (28%), and 40 g of additional water. The reactor was then sealed and heated to about 105° C. at which time the internal pressure had risen to about 10 psi. The contents of the reactor were held at this condition under constant stirring until all of the SMA had dissolved and the solution had reached a constant solids content (approximately 9.8%). An IR spectrum of the product at this point showed the presence of amide and carboxylate functionality. The product cured to a clear thermoset upon heating to 210° C. for 10 minutes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Substance count | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com