Compositions and Methods for Controlling Insects
a technology of insects and compositions, applied in the field of insects control, can solve the problems of inability to identify non-target species, use of toxic or deleterious effects on mammals, fish, fowl or other species, and difficulty in identification, and achieve the effect of repelling insects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Stably Transfected Schneider Cell Lines with Tyramine Receptor (TyrR)
[0057] A. PCR Amplification and Subcloning Drosophila melanogaster Tyramine Receptor
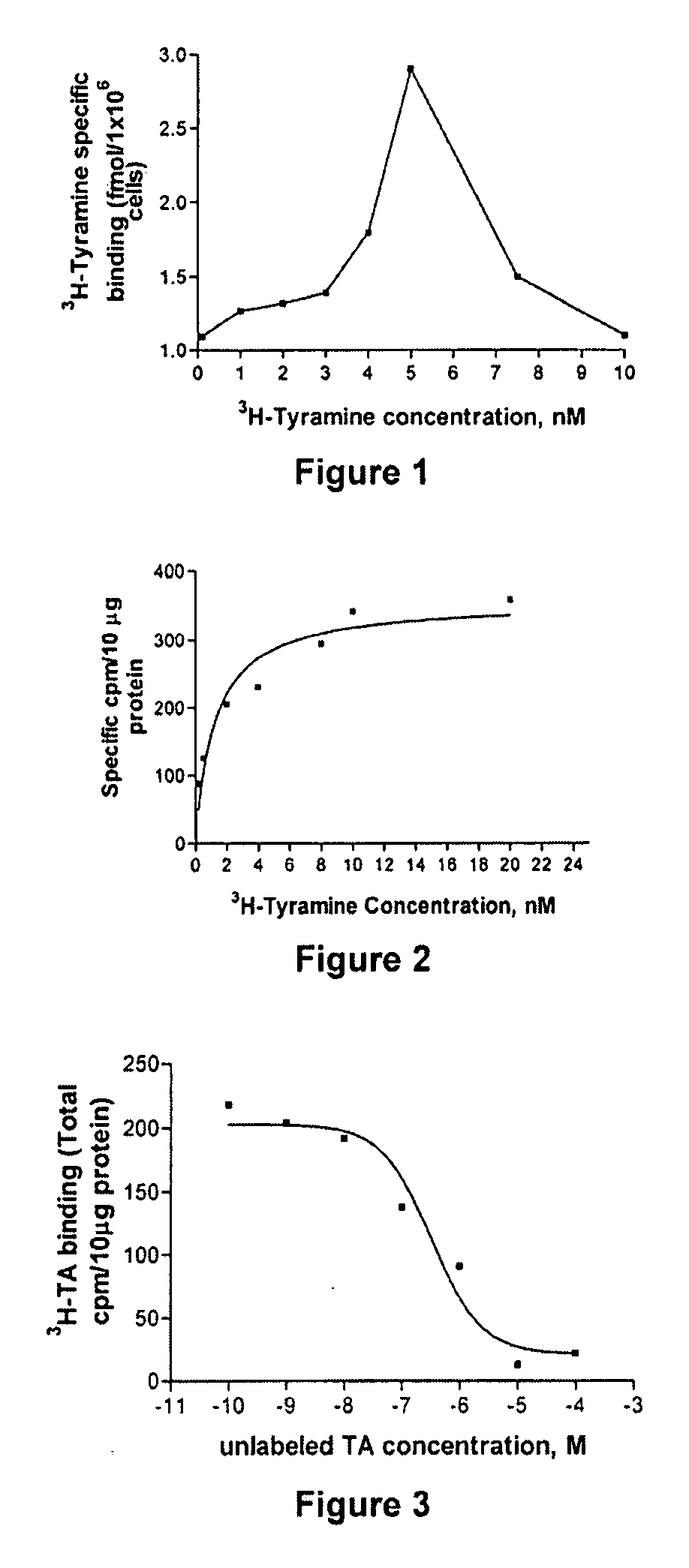
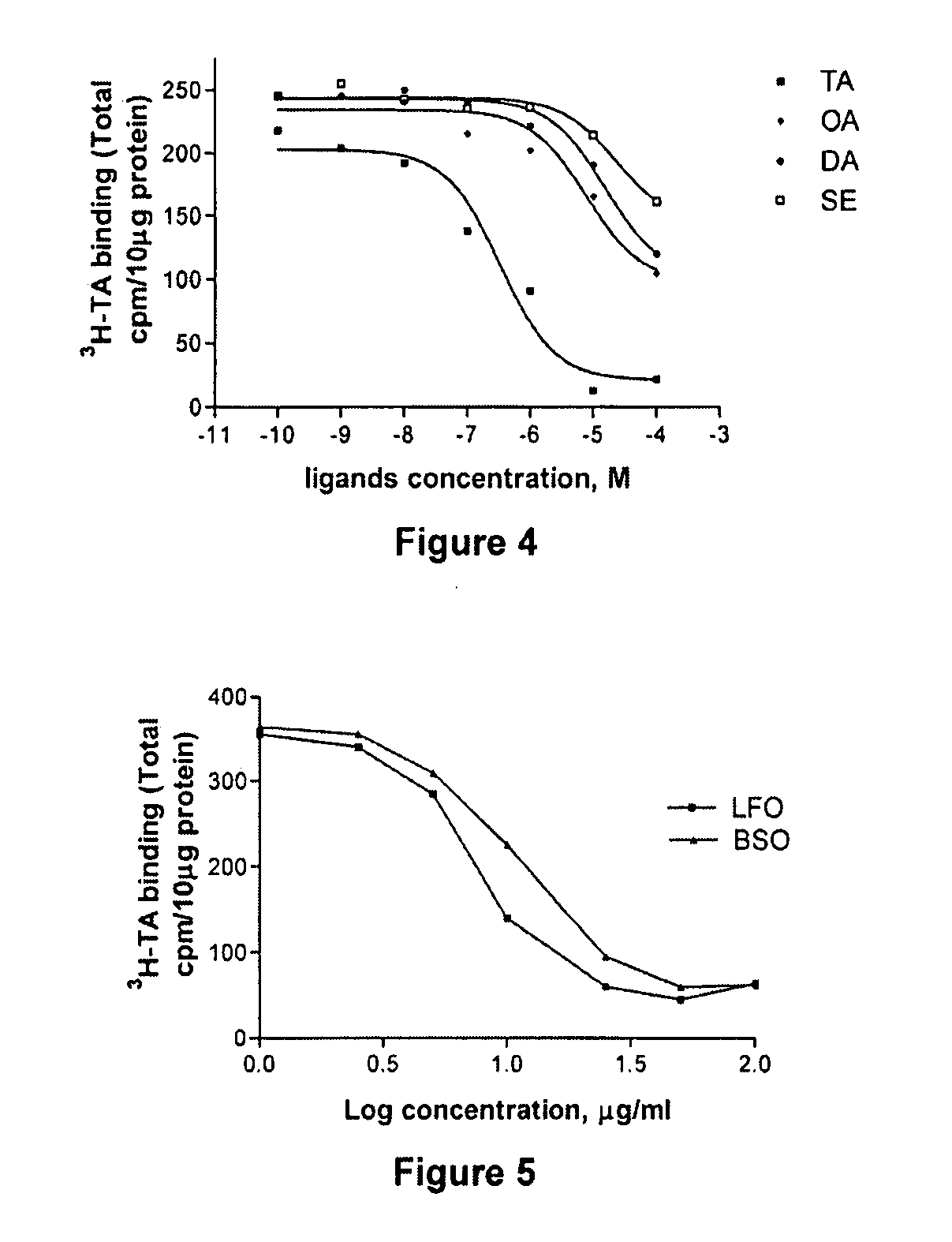
[0058] Tyramine receptor is amplified from Drosophila melanogaster head cDNA phage library GH that is obtained through the Berkeley Drosophila Genome Project (Baumann, A., 1999, Drosophila melanogaster mRNA for octopamine receptor, splice variant 1B NCBI direct submission, Accession AJ007617). The nucleic acid sequence and the peptide sequence of TyrR are set forth in SEQ ID NO: 1 and SEQ ID NO: 2, respectively. Phage DNA is purified from this library using a liquid culture lysate. (Baxter, et al., 1999, Insect Biochem Mol Biol 29, 461-467). Briefly, oligonucleotides that are used to amplify the open reading frame of the Drosophila tyramine receptor (TyrR) (Han, et al., 1998, J Neurosci 18, 3650-3658; von Nickisch-Rosenegk, et al., 1996. Insect Biochem Mol Biol 26, 817-827) consist of the 5′ oligonucleotide: 5′gccga...
example 2
Treatment of Cells Expressing the Tyramine Receptor and Effect of Compositions on Intracellular [cAMP]
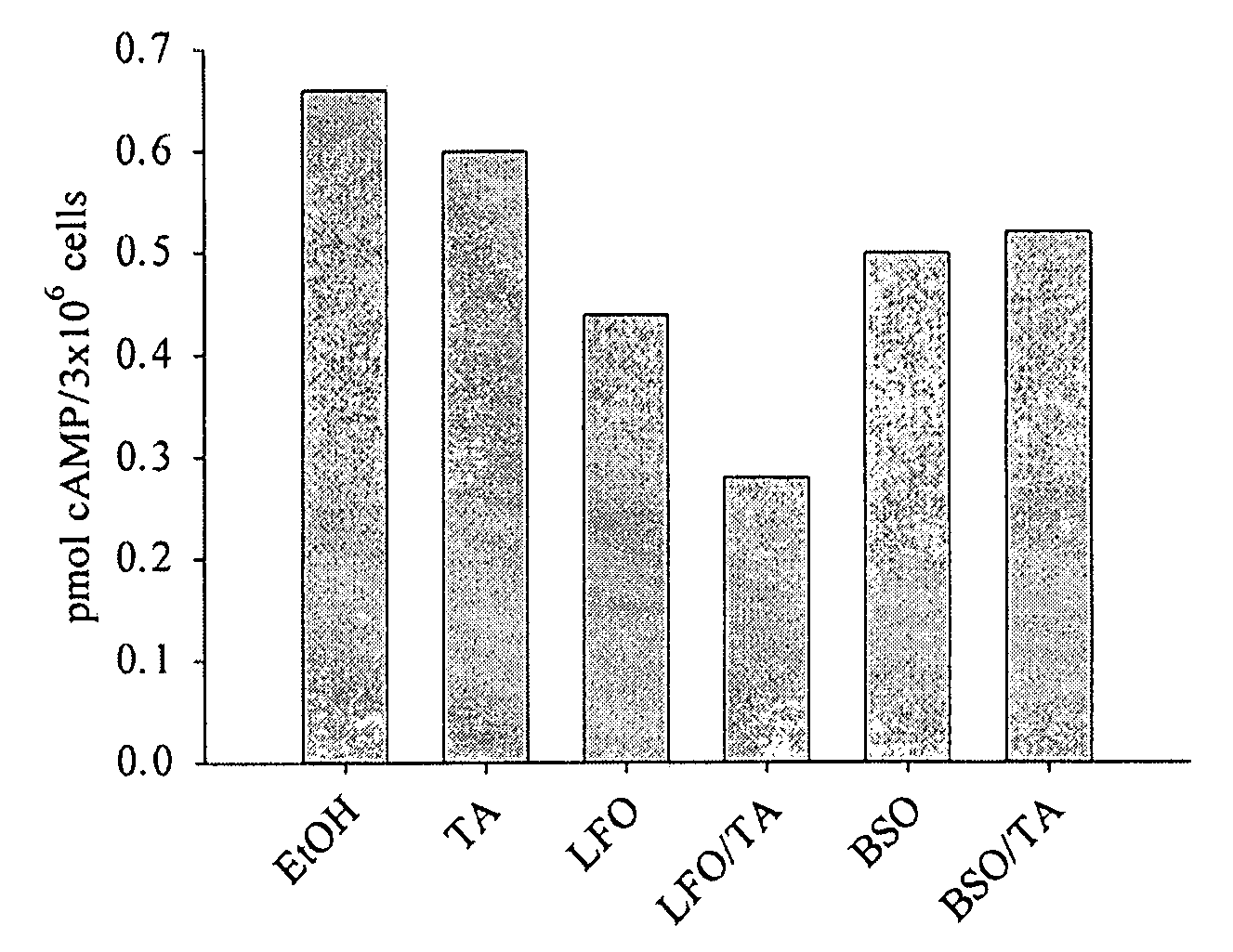
[0073] Cells are grown on dishes and the media changed the day before the treatment. When cells are approximately 95% confluent, media is aspirated and the cells are washed one time with about 5 mL of about 27° C. insect saline (170 mM NaCl, 6.0 mM KCl, 2.0 mM NaHCO3, 17.0 mM glucose, 6.0 mM NaH2PO4, 2.0 mM CaCl2, 4.0 mM MgCl2; pH 7.0). About 20 mL of insect saline is added, and cells are harvested by gentle scraping. An aliquot of the cells is counted by hemocytometer, and the cells are then centrifuged for about 5 minutes at about 1000 RPM. Cells are resuspended to give about 3×106 cells per mL. IBMX is added to about 200 μM. Then about 1 mL of cell suspension is aliquoted for treatment. Forskolin (cAMP inducing agent), tyramine or different composition candidates are added, and the cells are incubated at about 27° C. for about 10 minutes.
[0074] Treated cells are centrifuged at a...
example 3
Treatment of Cells Expressing the Tyramine Receptor and Effect of Compositions on Intracellular [Ca2+]
[0076] Intracellular calcium ion concentrations ([Ca2+]i) are measured by using the acetoxymethyl (AM) ester of the fluorescent indicator fura-2 (Enan, et al., Biochem. Pharmacol vol 51, 447-454). In this study, cells expressing tyramine receptor are grown under standard conditions. A cell suspension is prepared in assay buffer (140 mM NaCL, 10 mM HEPES, 10 mM glucose, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2) and cell number adjusted to about 2×106 cells per ml. Briefly, about 1.0 ml cell suspension (about 2×106 cells) is incubated with about 5 μM Fura 2 / AM for about 30 min at about 28° C. After incubation, the cells are pelleted at about 3700 rpm for about 10 sec at room temperature and then resuspended in about 1.5 ml assay buffer. [Ca2+]i changes are analyzed in spectrofluorometer in the presence and absence of test chemicals. Excitation wave lengths are about 340 nm (generated by Ca2+-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| emission wave length | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com