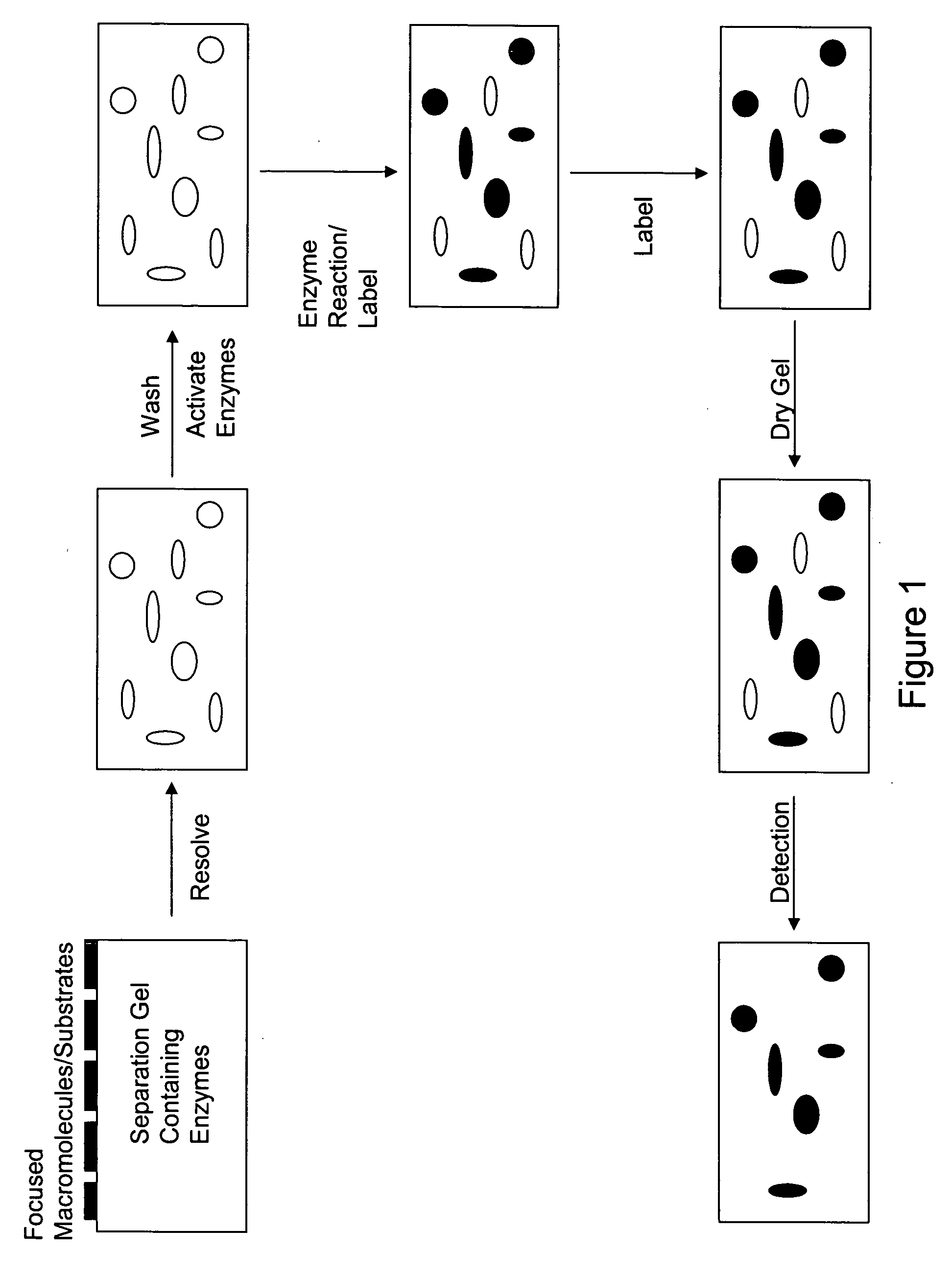
Devices and methods for profiling enzyme substrates
a technology of enzyme substrates and devices, applied in the field of apparatus and methods for separating and detecting enzyme substrates, can solve the problems of unknown substrate phosphorylation spectrum and unwanted side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0077]CK2 regulates the half-life of the prostate-specific tumor suppressor NKX3.1 (Li, X., et al., Mol Cell Biol, 26:3008-3017 (2006)). Only the free monomeric form of one catalytic subunit, CK2α′, is involved in regulating NKX3.1.
Purification of CK2 and Demonstration of Enzyme Activity
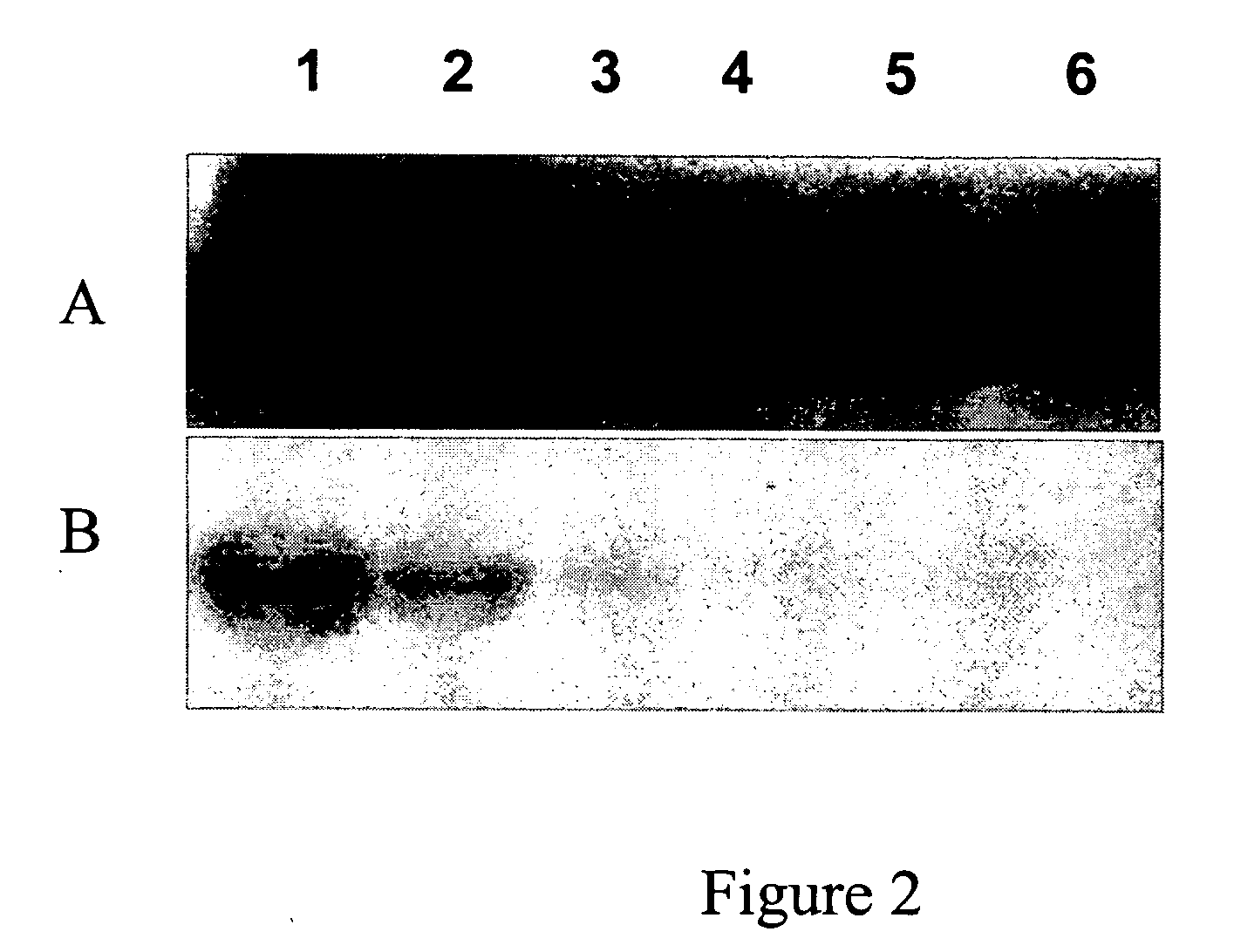
[0078]The protein coding regions of human CK2α gene was cloned into a protein expression vector to produce the protein in E. coli. A CK2α kinase activity-deficient mutant was also generated in parallel. CK2 α was expressed in E. coli and partially purified by nickel resin chromatography. To confirm that the recombinant CK2 α possessed kinase activity that could be manifested after electrophoresis in a denaturing polyacrylamide gel, an in-gel kinase assay was performed on the partially purified CK2 α with casein as a substrate in the gel. Standard conditions for SDS removal (i.e., alcohol-comprising buffer), protein refolding, and a kinase reaction were used. An exemplary pro...
example 2
Detection of CK2 Substrates in an LNCaP Cell Extract by ID RIKA and KESTREL
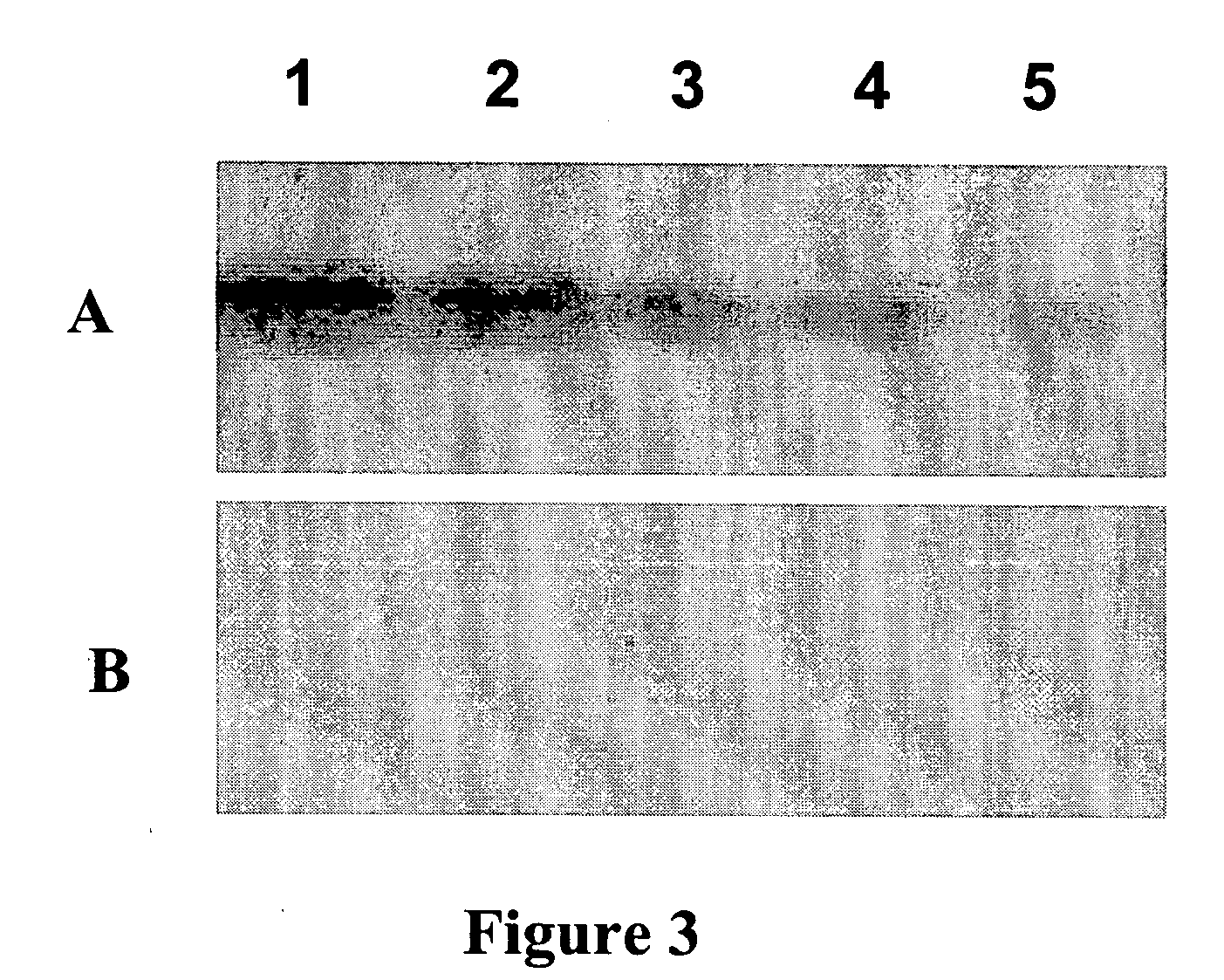
[0110]To compare the extent to which RIKA and Kinase Substrate Tracking and Elucidation System (KESTREL) are capable of detecting potential substrates, a whole cell lysate from LNCaP cells (50 mgs protein) will be fractionated by anion exchange chromatography and eluted with a NaCl step gradient into 40 fractions. Equivalent volumes of the load, flow and elution fractions will then be analyzed in the RIKA and KESTREL analyses. To perform 1D CK2 RIKAs, two aliquots of each fraction will be treated with λ phosphatase and electrophoresed through denaturing acrylamide gels containing either 10 μg / ml active or kinase-dead CK2α. After electrophoretic resolution of proteins on the RIKA gel, SDS will be removed by incubation in 20% isopropanol. The gels will then be carried through a series of buffer changes to refold the proteins present in the gel and to restore the catalytic activity of CK2α. A kinase reaction wil...
example 3
Validation the RIKA by Demonstrating Its Ability to Detect Known CK2 Substrates and to Discover Previously Unknown Substrates
Mass Spectrometric Identification of Potential CK2α Substrates Revealed by RIKA.
[0116]Since the total number of true CK2 targets in human cells is unknown, it is difficult to derive a statistical argument to determine how many identifications should be performed to achieve a given degree of confidence. It is anticipated that by characterizing 80-100, likely both known and unknown potential substrates will be encountered. To achieve this goal, a series of 2D CK2α RIKAs will be performed using fractionated LNCaP extracts as a source of potential CK2α substrates
[0117]Aliquots of anion exchange fractions of LNCaP whole cell lysates will be focused on 18 cm linear IPG strips with a pH range of 3-6, 5-8, 7-9, and 3-10. Preliminary studies using pH 3-10 IPG strips demonstrated that the majority of potential targets lie within the range of pH 3-5. It is anticipated th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com