Purine and Pyrimidine Cdk Inhitbitors and Their use for The Treatment of Autoimmune Diseases
a technology of pyrimidine cdk and autoimmune diseases, which is applied in the direction of biocide, plant growth regulators, pharmaceutical non-active ingredients, etc., can solve the problems of abnormal growth of an organ, changes in organ function, and destruction of one or more types of body tissues, and many of the treatments available to date are associated with severe adverse side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples 1 and 2
Materials and Methods
Proteinuria and Renal Function
[0173]Urinary protein concentration was determined by the Coomassie blue G dye-binding assay with bovine serum albumin as standard. Renal function was assessed as BUN in heparinized blood by the Reflotron test (Roche Diagnostics Corporation, Indianapolis, USA). BUN levels exceeding 30 mg / dl were considered abnormal (normal range in this laboratory for mice: 14-29 mg / dl).
Anti-DNA Antibodies
[0174]The levels of anti-dsDNA autoantibodies were evaluated in the serum by an enzyme-immunoassay (Diastat anti-ds DNA kit, Bouty Laboratory, Milano, Italy) as described before (Kidney Int, 53:726-734, 1998).
[0175]Serum levels of AST and ALT were measured using an autoanalyzer (CX5, Beckman Instruments Inc., Fullerton, Calif.).
Renal Morphology
[0176]Light microscopy: Fragments of renal cortex were fixed in Dubosq-Brazil, dehydrated in alcohol and embedded in paraffin. Sections (3 μm) were stained with hematoxylin and eosin, Masson...
example 1
Results
Body Weight, Food and Water Intake
[0180]As shown in Table 1 lupus mice gained weight during the study. No difference in body weight was observed among the experimental groups. Food (Table 2) and water (Table 3) intake evaluated every two weeks from 2 to 5 months were comparable among vehicle and CYC202 treated mice.
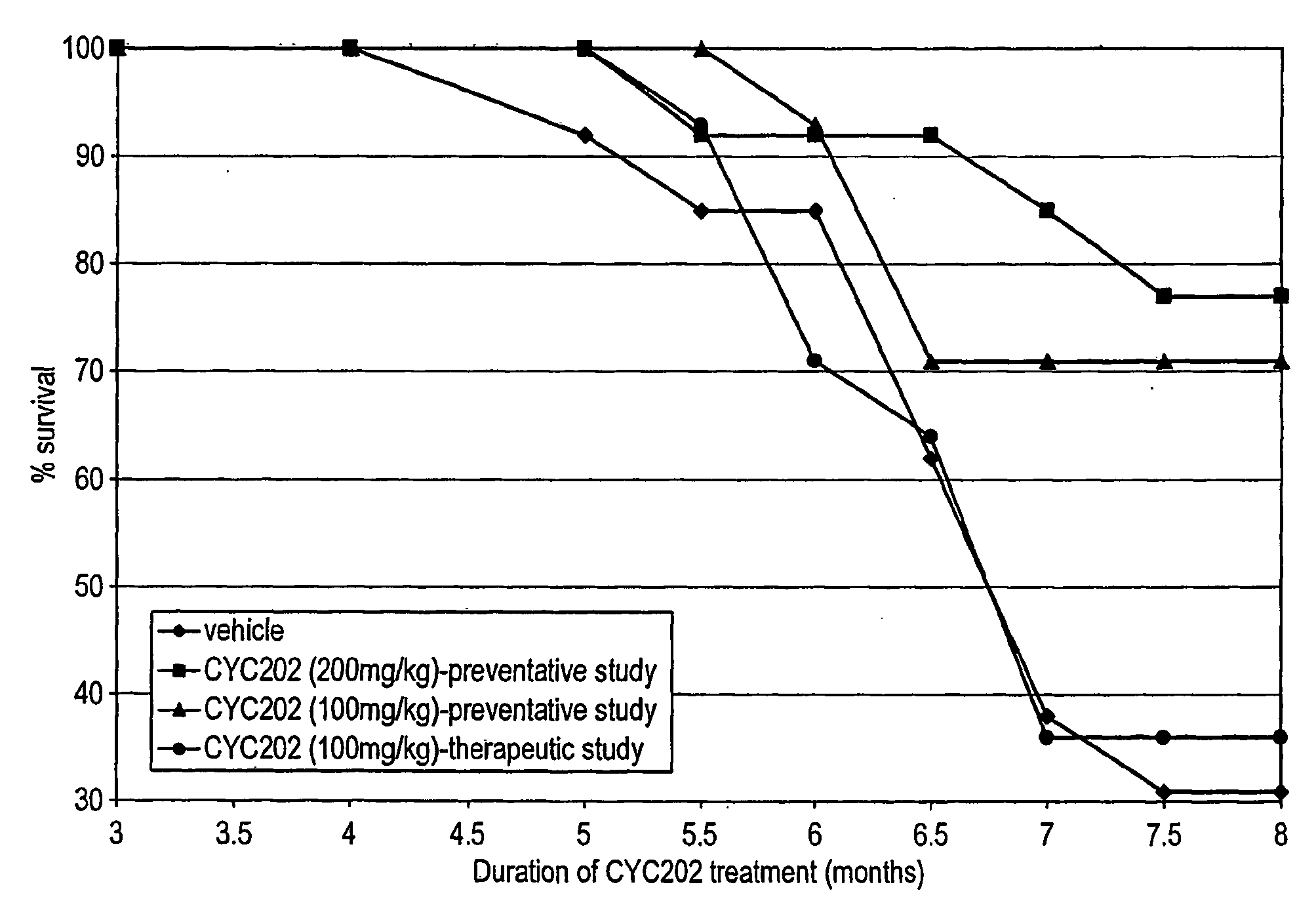
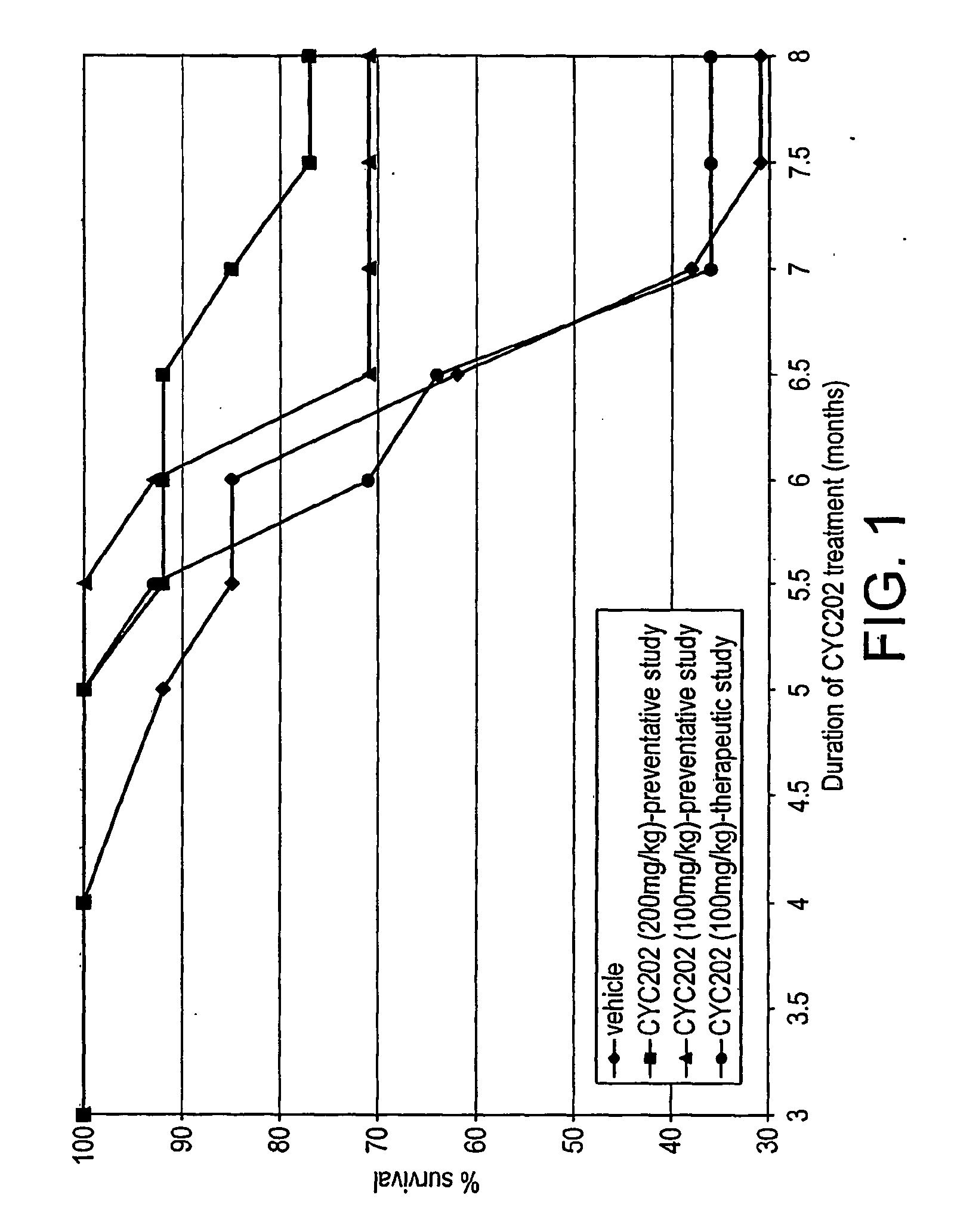
Lupus Mice Survival
[0181]NZB / W F1 mice treated with CYC202 at the doses of 200 and 100 mg / kg, starting from 2 months of age, survived significantly (P<0.05) longer than vehicle-mice (see Table 4 and FIG. 1). Actually, at the end of the study (month 8) while only four of thirteen NZB / W mice (31%) that had been treated with vehicle were alive, ten of thirteen mice (77%) and ten of fourteen mice (71%) treated with 200 and 100 mg / kg CYC202, respectively, survived. In the group of mice given CYC202 (100 mg / kg) from 5 months of age (therapeutic treatment) the percentage of survival was not different from that recorded in vehicle-mice.
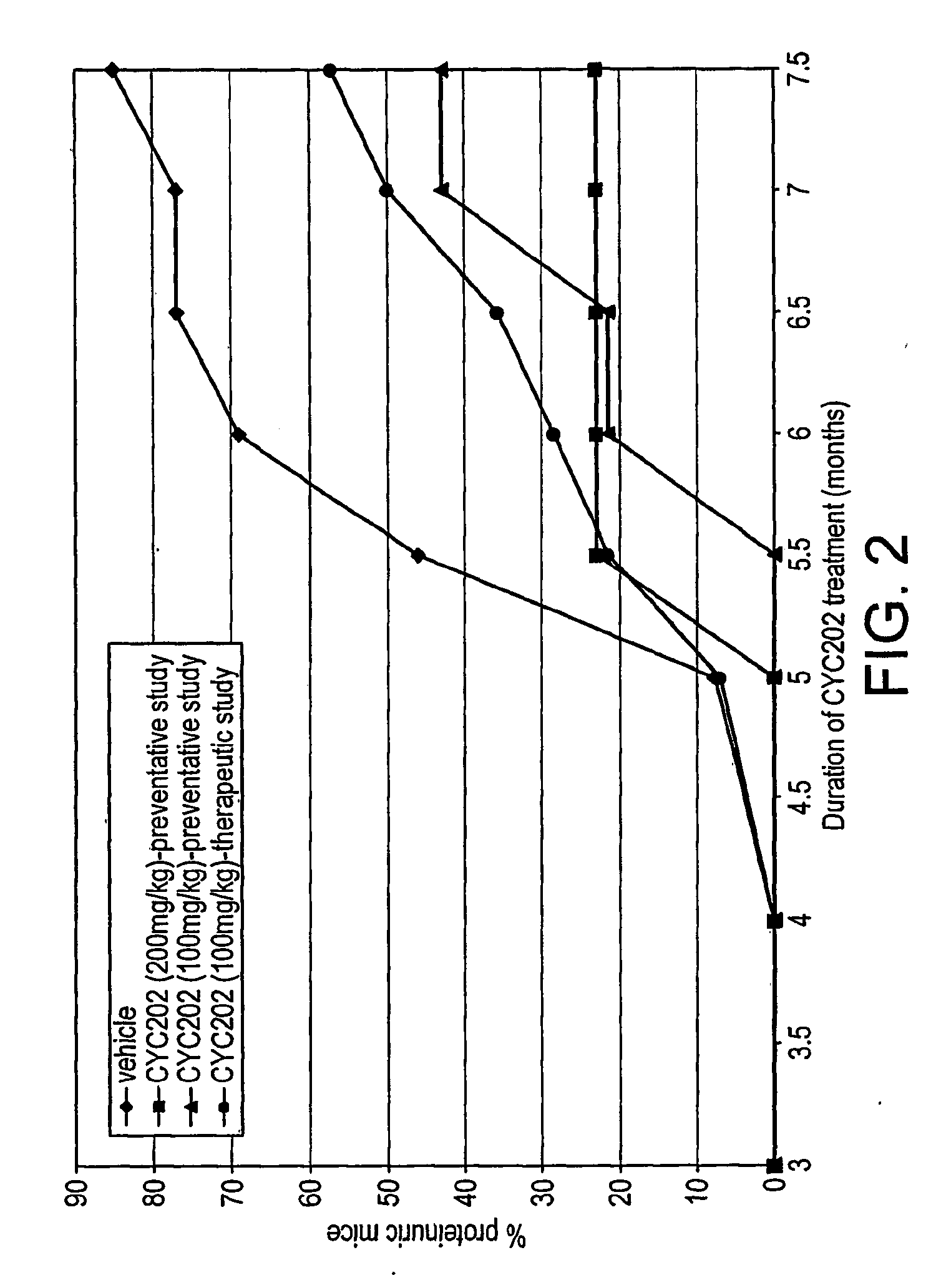
Proteinuria and Renal Function
[0182]C...
example 2
Results
[0190]NZB / W F1 mice gained weight during the study. No difference in body weight was observed among the experimental groups.
Survival
[0191]NZB / W F1 mice treated with the combination of CYC202 and methylprednisolone (MPS), starting from 5 months of age, survived significantly (P<0.0001) longer than vehicle-mice (Table 11). Notably, at 12 months, when all mice given vehicle died, ten of sixteen animals (62%) treated with the combined therapy were alive. Survival curves of mice receiving the single therapies were not different from that of vehicle group.
[0192]Table 12 shows the cumulative percentage of mice with proteinuria >4 mg / day evaluated at different stages of the disease. The association of CYC202 and MPS significantly delayed the onset of proteinuria compared to vehicle. In the interval from 7 to 10 months the proportion of proteinuric mice in the combined therapy group was significantly lower than in the vehicle group (6.2 to 43.8% versus 40 to 90%)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com