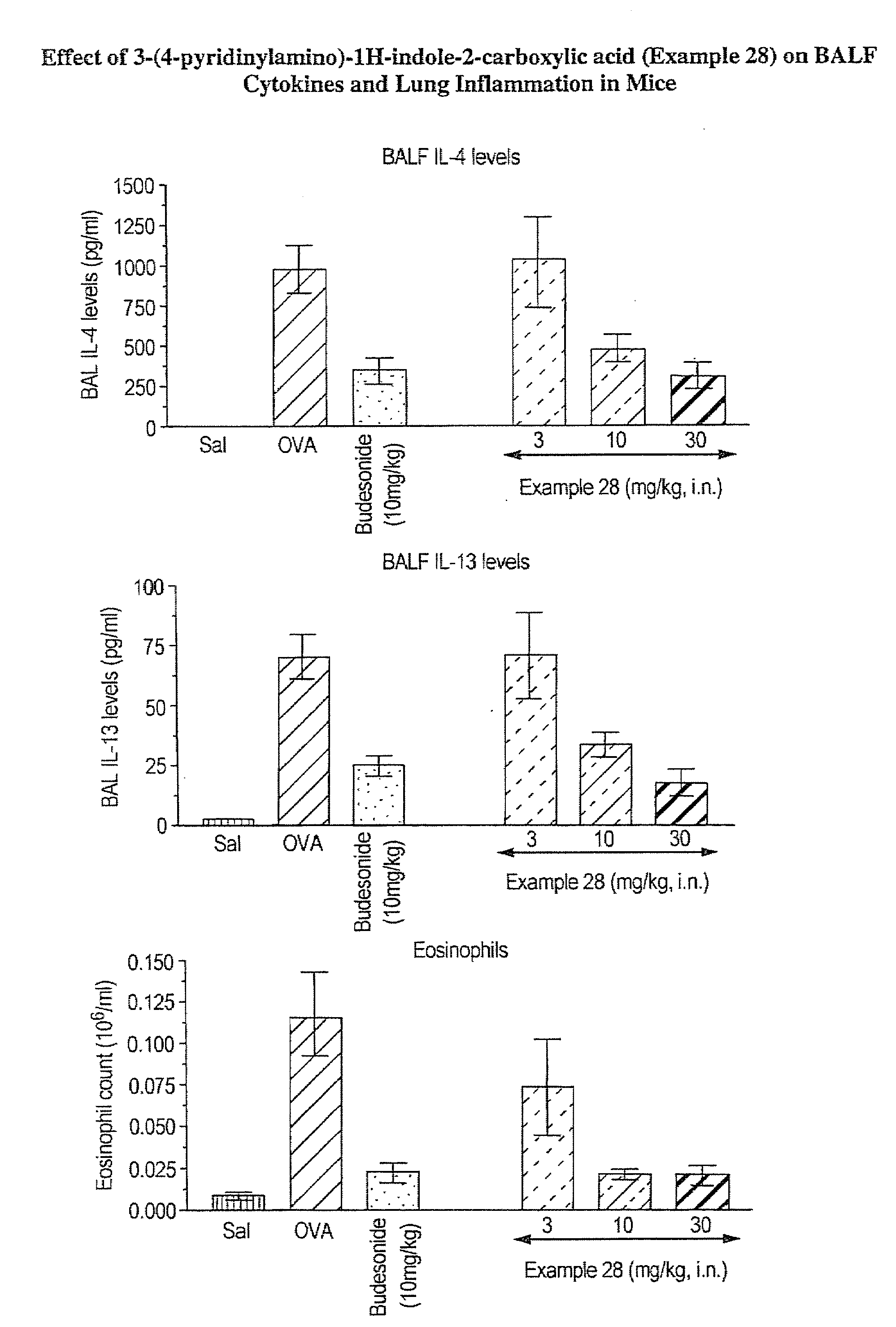
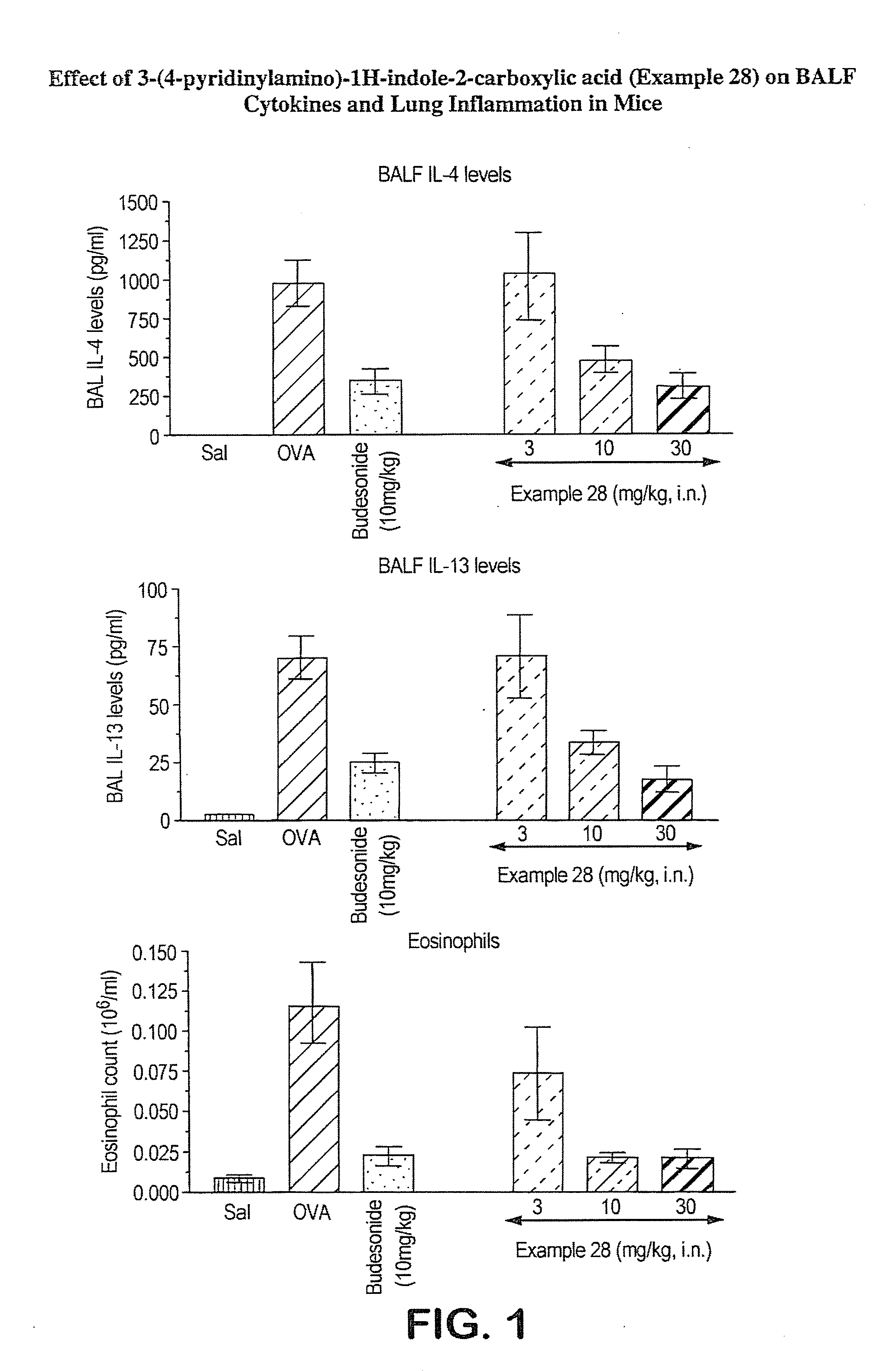
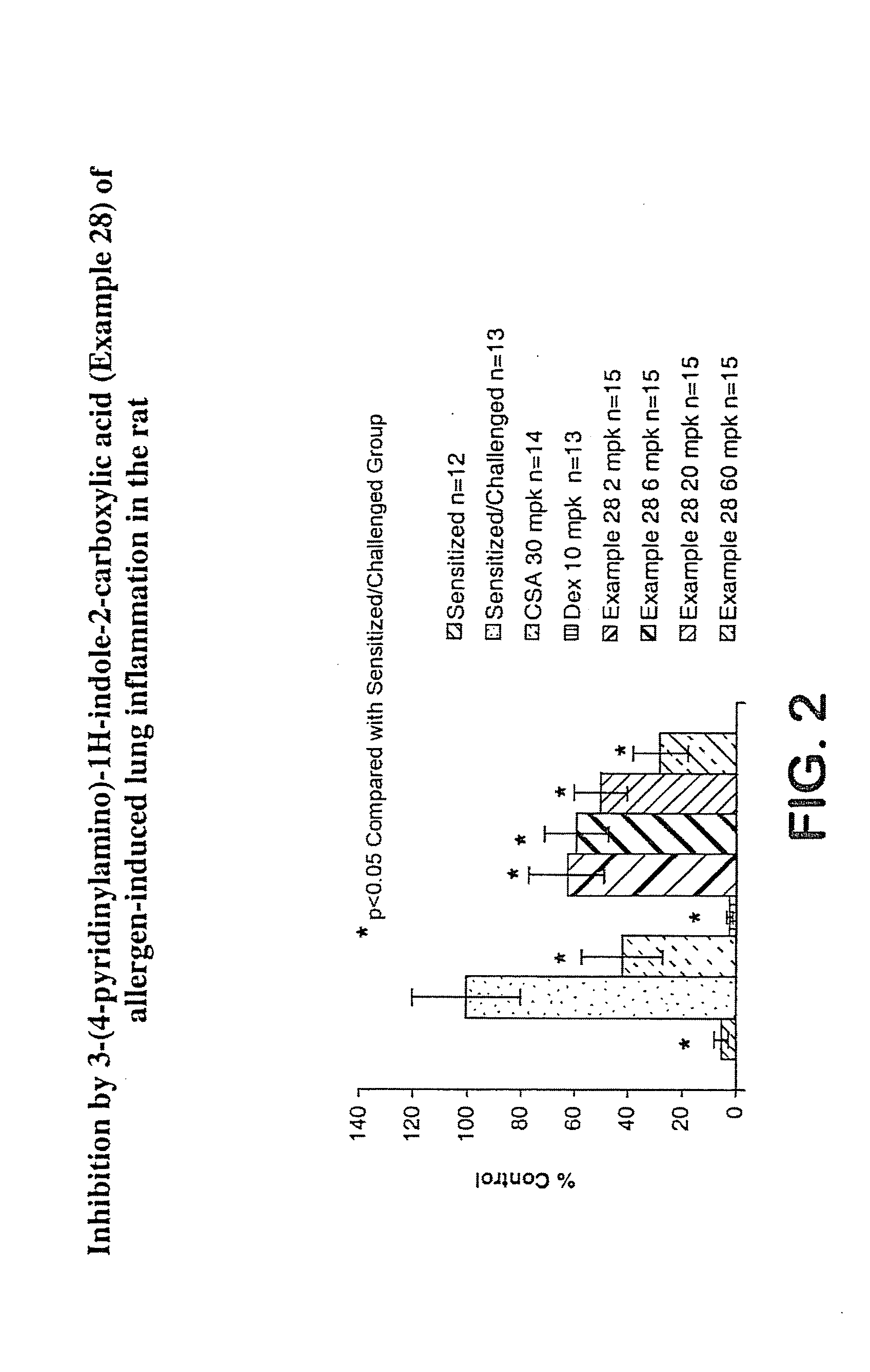
Interleukin-4 gene expression inhibitors
a technology of interleukin-4 and gene expression inhibitor, which is applied in the direction of antibacterial agents, immunological disorders, drug compositions, etc., can solve the problems of inflammation of the lower airway, additional health risks of overactivation of th2 cytokines,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0389]
Scheme A, Step A1: 3-Amino-6-trifluoromethyl-1H-indole-2-carboxylic acid, ethyl ester
[0390]Under nitrogen, stir and heat a mixture of 2-fluoro-4-(trifluoromethyl)benzonitrile (1.0 g, 5.29 mmol), ethyl glycinate hydrochloride (886 mg, 6.3 mmol), potassium carbonate (1.46 g, 6.3 mmol) and 1-methyl-2-pyrrolidinone (20 mL) at 115-120° C. After six hours add potassium tert-butoxide (700 mg, 6.2 mmol), and stir at ambient temperature for 2 h. Quench into ice / water, extract the aqueous mixture with ether, wash the extract with water and dry it with magnesium sulfate. Filter and concentrate under vacuum to yield the crude product. Chromatograph the crude product on silica gel (10 g, Sepack cartridge) with dichloromethane as eluent to afford 332 mg (23%) of the title compound. Identical on TLC (silica gel, dichloromethane) to the compound disclosed in U.S. Pat. No. 5,189,054.
example 2
[0391]
Scheme A, Step A1: 3-Amino-6-fluoro-1H-indole-2-carboxylic acid, ethyl ester
[0392]Under nitrogen, stir and heat a mixture of 2,4-difluorobenzonitrile (1.14 g, 8.2 mmol), ethyl glycinate hydrochloride (1.5 mg, 10.7 mmol), potassium carbonate (3.4 g, 24.6 mmol) and 1-methyl-2-pyrrolidinone (20 mL). After 2 h allow the reaction to cool to 40° C. and add potassium tert-butoxide (300 mg, 2.7 mmol). Allow the reaction to stir and for 1 h and then add additional potassium tert-butoxide (500 mg, 4.4 mmol). Cool to room temperature overnight, pour the reaction mixture onto ice. Add water to dissolve the solids in the reaction flask and add to the reaction quench. Filter off the precipitated brown solid and extract the filtrate with ethyl acetate. Wash the extract with water (2×'s), brine and dry over magnesium sulfate. Filter and concentrate under vacuum to give a residue, which is triturated with dichloromethane to afford a solid. Collect the solid, concentrate the filtrate and chroma...
example 3
[0393]
Scheme B: 3-Amino-5-chloro-1H-indole-2-carboxylic acid, ethyl ester
Step B1: 2-Amino-N-tert-butyl-5-chlorobenzamide
[0394]Stir and cool to 0° C. a solution of 2-amino-5-chlorobenzoic acid (1.21 g, 10 mmol) and N-hydroxysuccinimide (1.38 g, 12 mmol) in dichloromethane (12 mL). Add, dropwise, a 1M solution of dicyclohexylcarbodiimide in dichloromethane (12 mL, 12 mmol), and stir at 0° C. for 1 h. Add tert-butyl amine (1.74 g, 23.79 mmol) by syringe and stir at ambient temperature overnight. Filter the reaction mixture and wash the filtrate with aqueous sodium bicarbonate. Dry the organic phase with MgSO4 and chromatograph over silica gel eluting with 4% ethyl acetate / dichloromethane to afford 1.74 g of the title compound: MS 227 (M+H), m.p. 126-127° C.
Step B2: N-(4-Chloro-2-cyanophenyl)-2,2,2-trifluoroacetamide
[0395]Stir and cool to 0° C. a solution 2-amino-N-tert-butyl-5-chlorobenzamide (5.07 g, 22.36 mmol) in dichloromethane (200 mL), and add, dropwise trifluoroacetic anhydride....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com