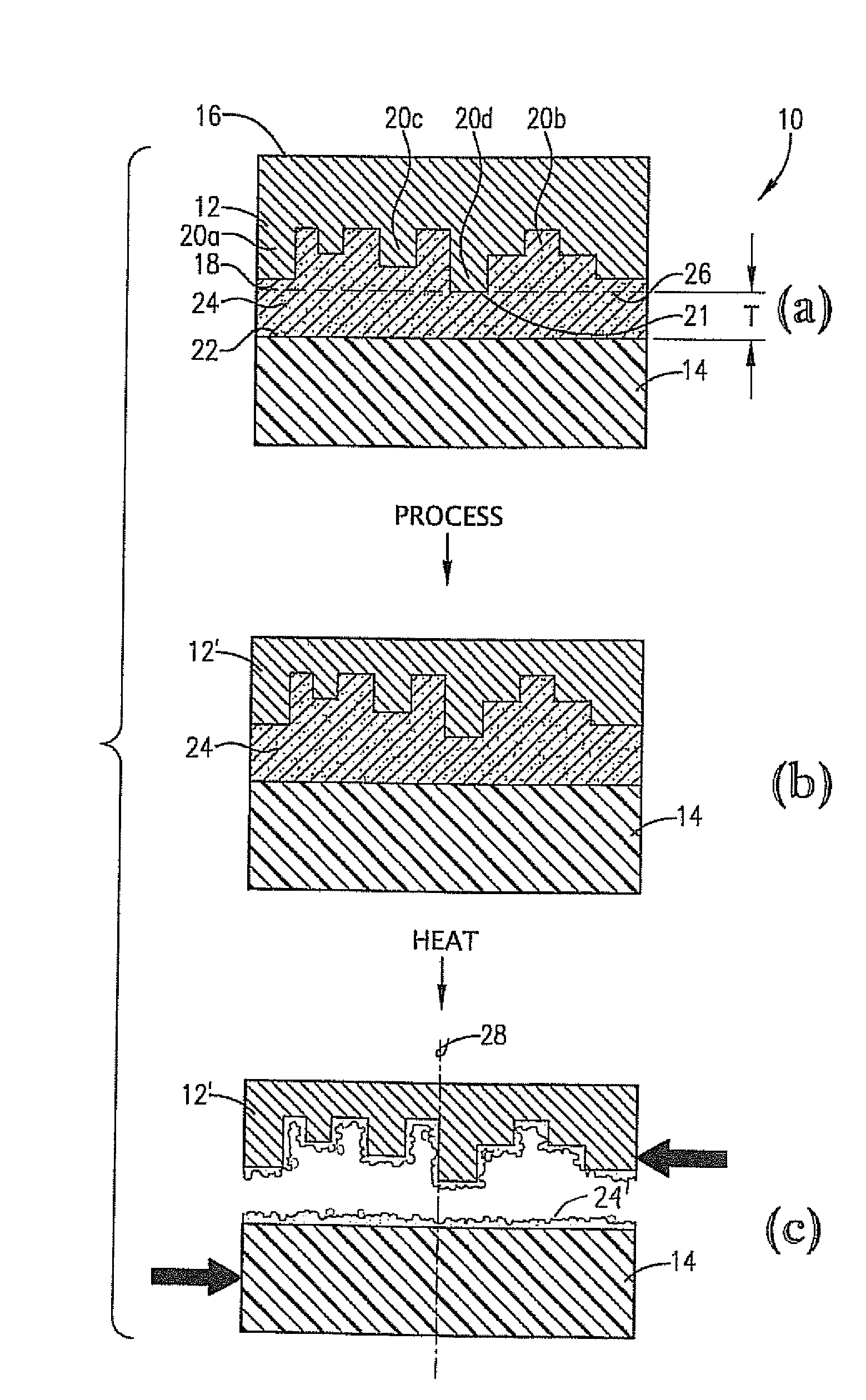
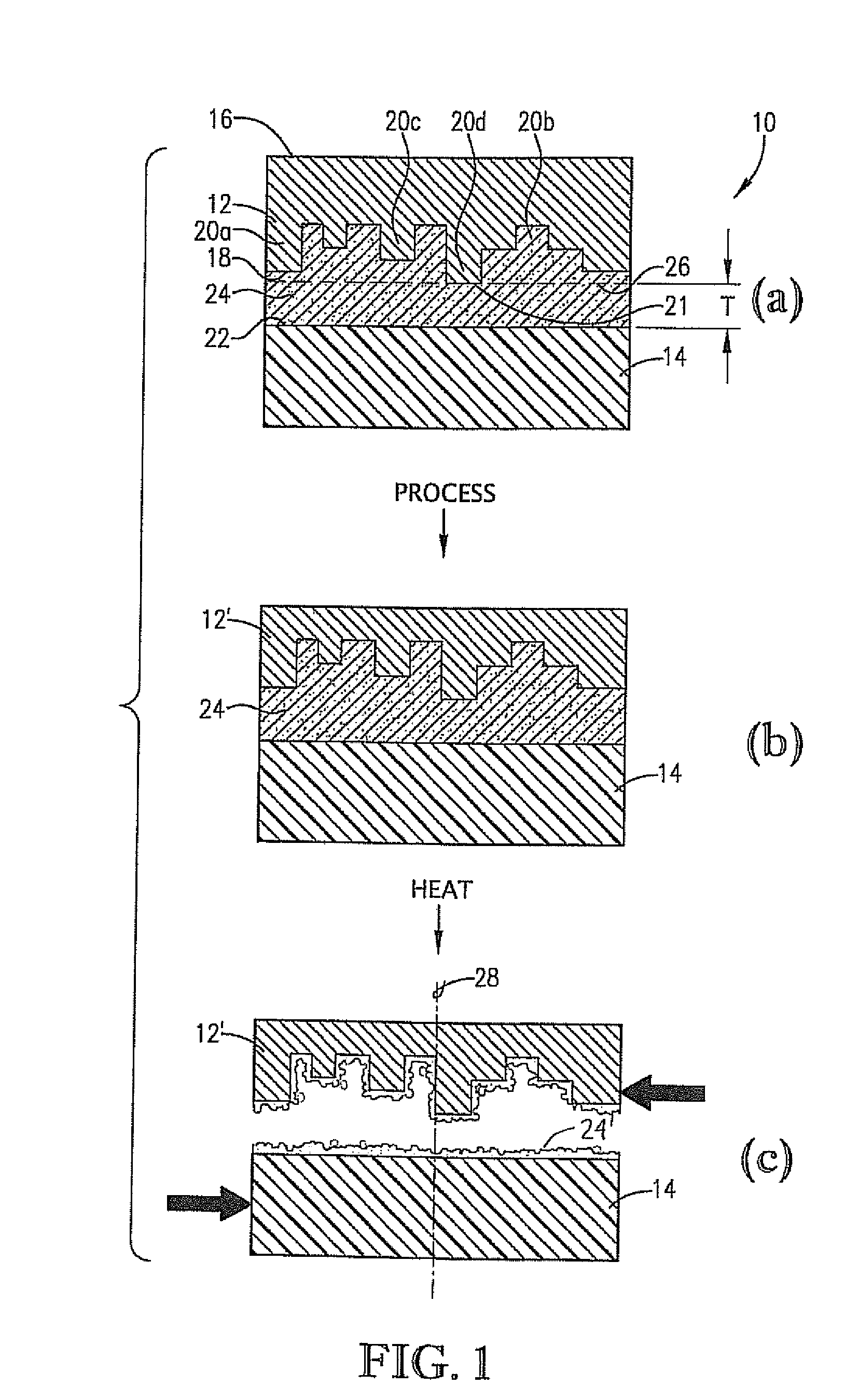
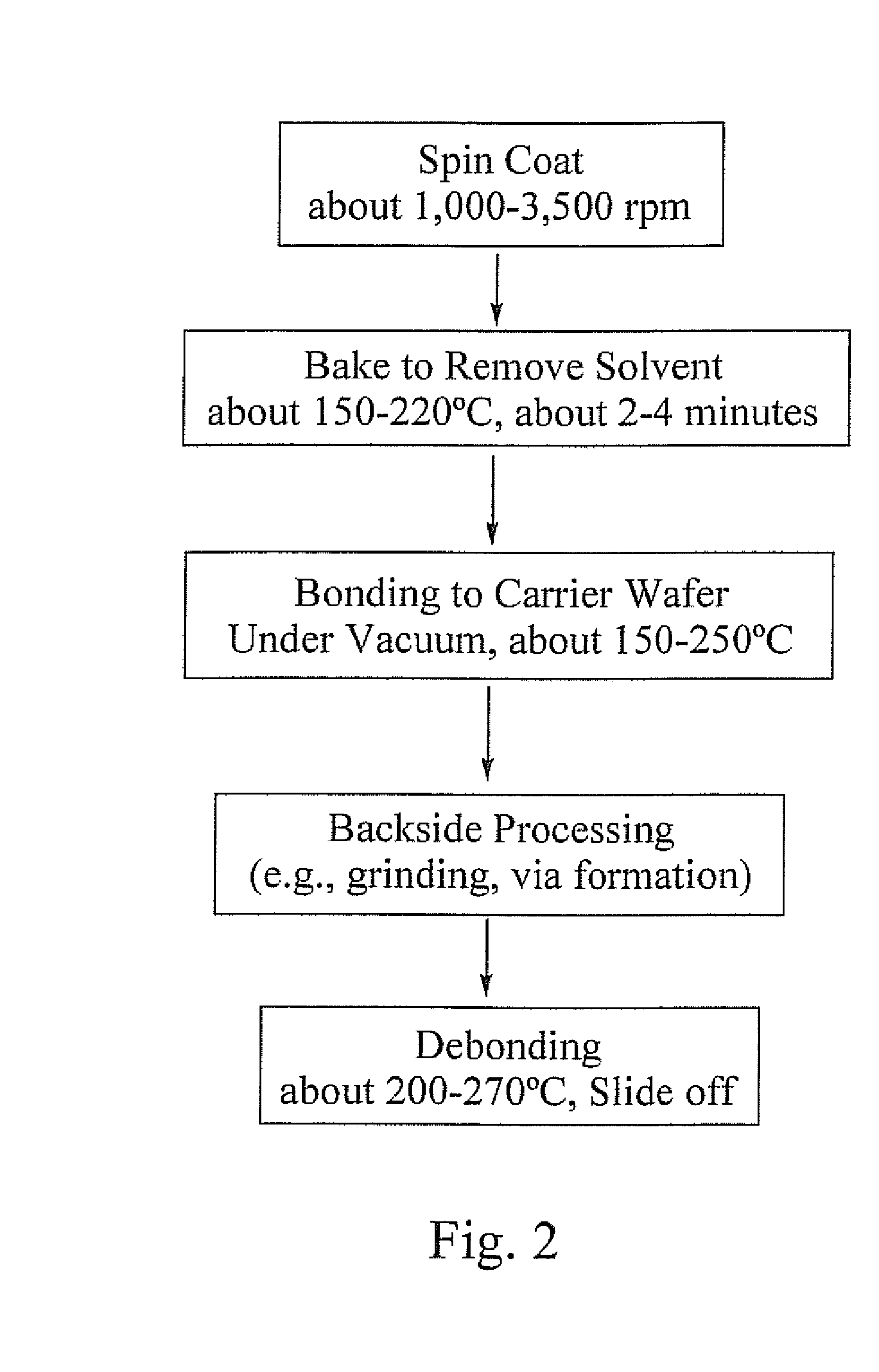
High-temperature, spin-on, bonding compositions for temporary wafer bonding using sliding approach
a technology of bonding compositions and wafers, applied in the direction of layered products, transportation and packaging, chemistry apparatuses and processes, etc., can solve the problems of increasing capacitance, requiring thicker transmission lines, and large ic footprin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0056]Formulations were made by dissolving various cellulose derivatives (obtained from Eastman Chemical Company, Kingsport, Tenn.) in appropriate solvents. The exact materials and quantities used are reported in Table I.
TABLE IBonding Composition Formulations from Cellulose Materials.SAMPLESAMPLESAMPLESAMPLE1.11.21.31.4INGREDIENTS(g)(g)(g)(g)Cellulose acetate (29.5%)20000butyrate (17%)Cellulose acetate01800trimelliateCellulose acetate (2%)00250butyrate (52%)Cellulose acetate (18.5%)00025butyrate (31%)Propylene glycol08200monomethyl etherMethyl isoamyl ketone5007550Ethyl acetoacetate300025
example 2
Cycloolefin Resin and Poly(α-Pinene) Blend
[0057]Formulations were made by dissolving Zeonex 480R resin (obtained from Zeon Chemicals, Louisville, Ky.) and / or poly(α-pinene) (obtained from Aldrich, Milwaukee, Wis.) and / or poly(β-pinene) (obtained from Aldrich, Milwaukee, Wis.) in D-limonene (obtained from Florida Chemical Company). Bis(trimethoxysilylethyl)benzene (obtained from Aldrich, Milwaukee, Wis.) was added as an adhesion promoter. The exact compositions of the formulations are reported in Table II.
TABLE IIBonding Composition Formulations from Poly(cycloolefin)and Pinene Materials.SAMPLESAMPLESAMPLESAMPLE2.12.22.32.4INGREDIENTS(g)(g)(g)(g)Zeonex 480R12055.946.0520Poly(α-pinene)014.330.70Poly(β-pinene)0005D-limonene280144.8138.1574.875Bis(trimethoxysilyl-0.50.2680.2680.125ethyl)benzene
example 3
Cycloolefin Resin and Rosin Ester Blend
[0058]The formulations were made by dissolving Zeonex 480R resin and Eastotac H142W (obtained from Eastman Chemicals, Kingsport, Tenn.) in a suitable solvent. Irganox 1010 and Irgafos 168 (obtained from Ciba Specialty Chemicals, Tarrytown, N.Y.) were added to one of the formulations to prevent thermal oxidation at high temperatures. Triton X-100 (obtained from Aldrich, Milwaukee, Wis.) was added to reduce de-wetting problems, and bis(trimethoxysilylethyl)benzene was added to promote adhesion. The exact compositions of the formulations are reported in Table M.
TABLE IIIBonding Composition Formulations Based onPoly(cycloolefin) and Rosin Ester Blends.SAMPLESAMPLESAMPLESAMPLE3.13.23.33.4INGREDIENTS(g)(g)(g)(g)Zeonex 480R35 g740Eastotac H142W753160D-limonene12303060Mesitylene000140Irganox 10100002Irgafos 1680001Triton X-1000001Bis(trimethoxysilyl-0001ethyl)benzene
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| softening temperature | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com