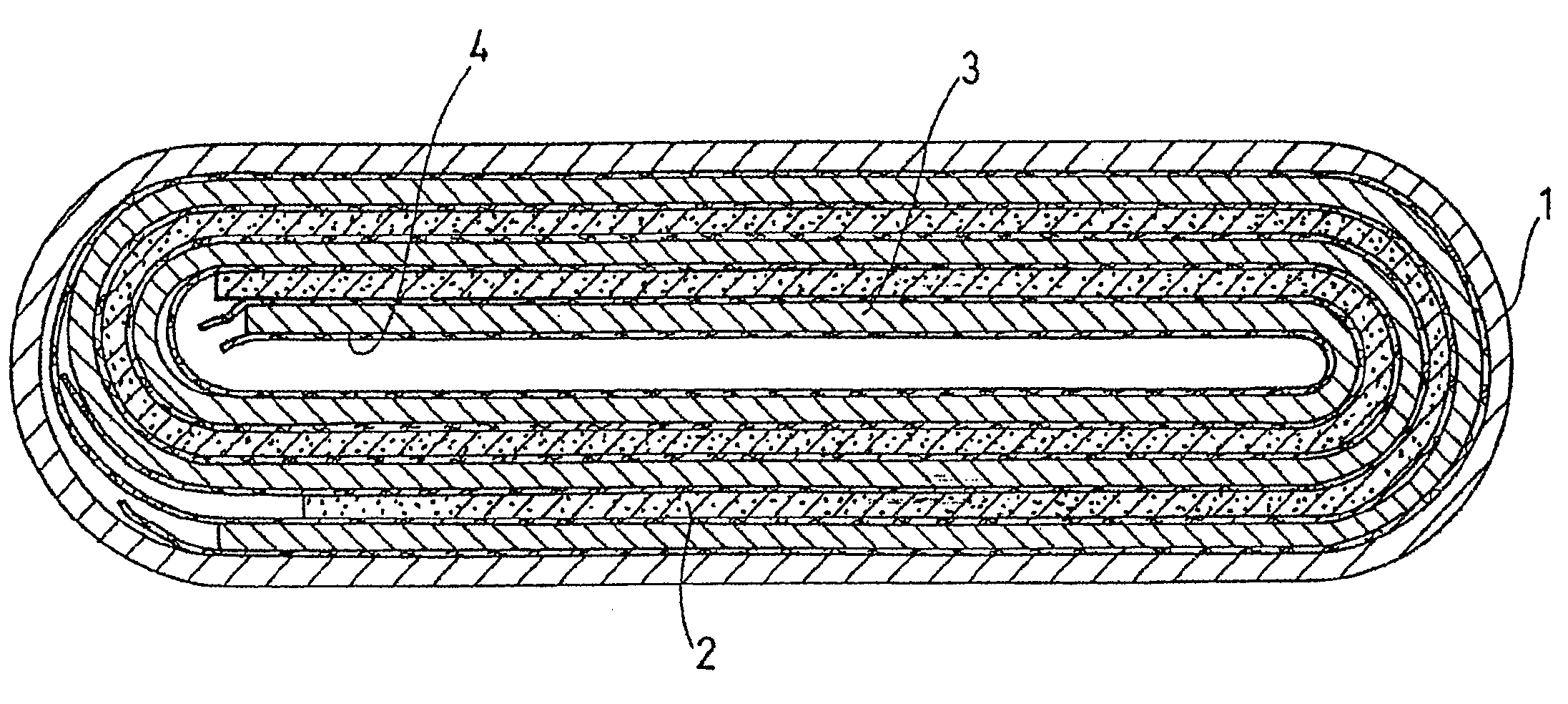
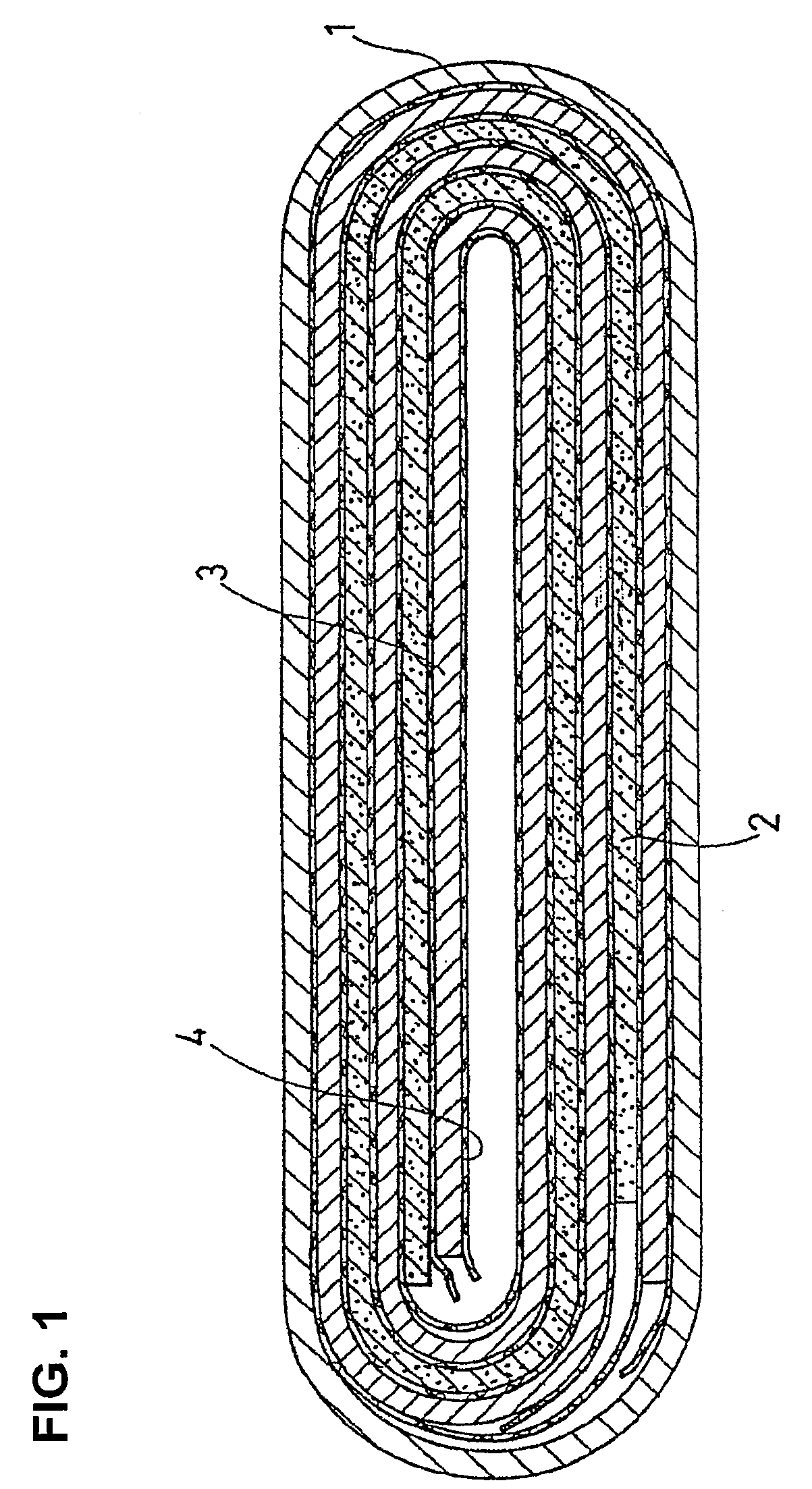
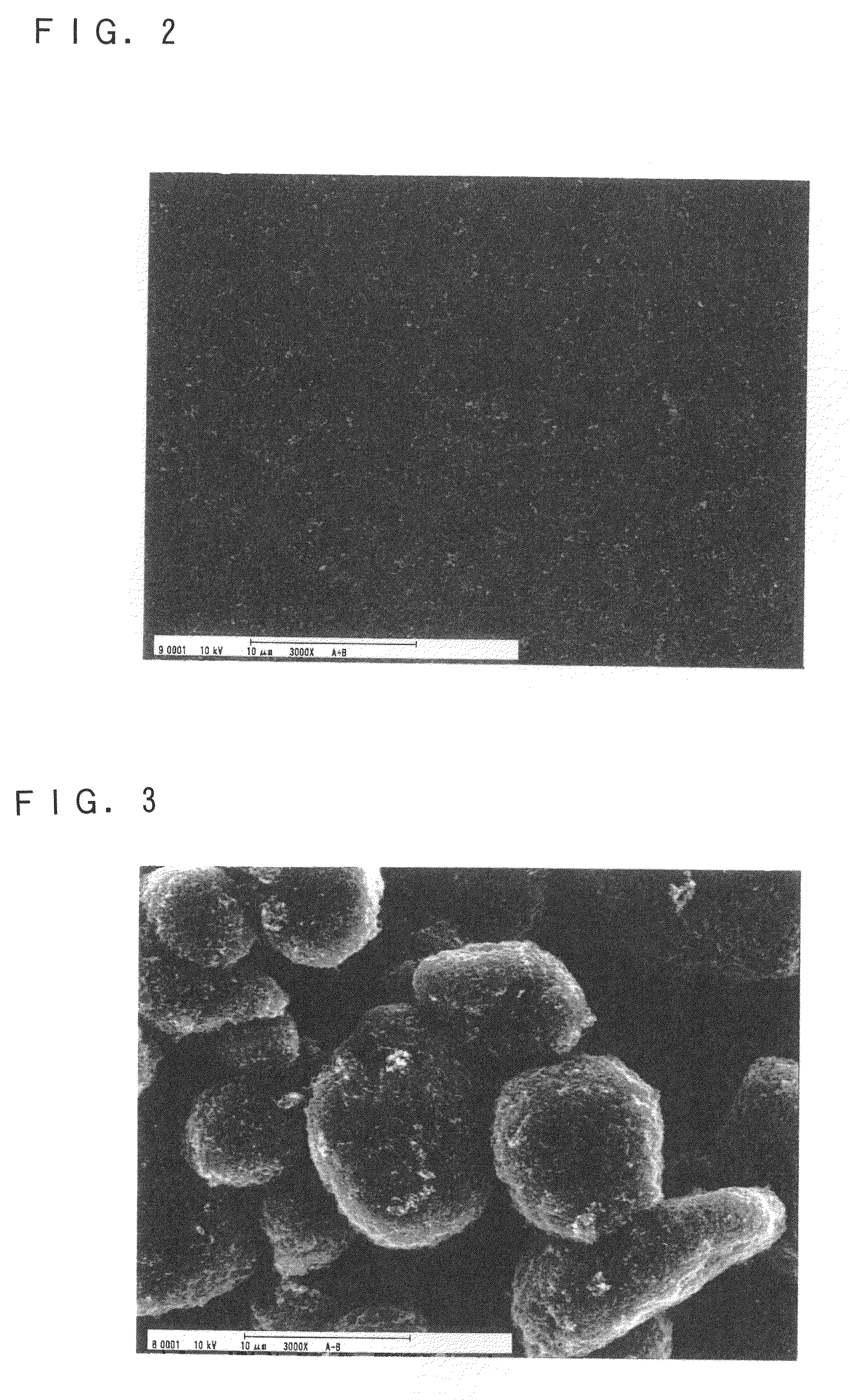
Non-aqueous electrolyte secondary battery and production method thereof
a technology of non-aqueous electrolyte and secondary batteries, which is applied in the direction of wound/folded electrode electrodes, cell components, sustainable manufacturing/processing, etc., can solve the problems of reducing discharge characteristics, electrode reactions, and conductive agents that tend to form agglomerated particles including fluorocarbon resin, so as to improve the active material density of positive electrodes, reduce the amount of conductive agents, and improve the effect of capacity and improved characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
(i) Production of Positive Electrode
[0097]The followings were measured for producing a positive electrode:
[0098]Active material: LiCoO2 (100 parts by weight)
[0099]Binder A: PVDF, which is a solid content of “KF polymer L #1320” manufactured by Kureha Chemical Industry Co., Ltd. (1 part by weight)
[0100]Binder B: hydrogenated nitrile rubber, which is a solid content of “BM-720H” manufactured by Nippon Zeon Co., Ltd. (0.5 part by weight)
[0101]Conductive agent: acetylene black having an average primary particle size of 0.03 μm (2 parts by weight)
[0102]LiCoO2 and PVDF (binder A) were kneaded using NMP as a dispersion medium to prepare an active material paste A having a solid content of 85 wt %.
[0103]Acetylene black and hydrogenated nitrile rubber (binder B) were kneaded using NMP as a dispersion medium to prepare a conductive agent paste B having a solid content of 22 wt %.
[0104]Part of the paste B was applied onto a glass plate to form a film having a thickness of 60 μm, and the film w...
examples 2 to 6
[0118]Positive electrodes and non-aqueous electrolyte secondary batteries were produced in the same manner as in Example 1 except that the amounts of the binders were varied as shown in Table 1.
example 7
[0119]A positive electrode and a non-aqueous electrolyte secondary battery were produced in the same manner as in Example 4 except for the addition of 0.3 part by weight of modified acrylic rubber fine particles per 100 parts by weight of the active material to the active material paste A in the first step. The modified acrylic rubber fine particles were insoluble in NMP and were solid content of “BM-500B” having an average particle size of 0.2 μm, manufactured by Nippon Zeon Co., Ltd.
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com