Iron composite particles for purifying soil or ground water, process for producing the same, purifying agent containing the same, process for producing the purifying agent and method for purifying soil or ground water
a technology of iron composite particles and soil, which is applied in the direction of water/sewage treatment by oxidation, separation processes, grain treatment, etc., can solve the problems of ineffective pcb treatment methods, production and use of dioxins having an extremely high toxicity to human bodies even in a trace amount, and the current prohibition of pcb treatmen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Production of Purifying Iron Composite Particles and Purifying Agent
[0211]A reaction vessel maintained under a non-oxidative atmosphere by flowing N2 gas at a rate of 3.4 cm / s, was charged with 704 L of a 1.16 mol / L Na2CO3 aqueous solution, and then with 296 L of an aqueous ferrous sulfate solution containing 1.35 mol / L of Fe2+ (amount of Na2CO3: 2.0 equivalents per equivalent of Fe), and the contents in the reaction vessel were reacted with each other at 47° C. to produce FeCO3.
[0212]The aqueous solution containing the thus obtained FeCO3 was successively held at 47° C. for 70 min while blowing N2 gas thereinto at a rate of 3.4 cm / s. Thereafter, air was passed through the FeCO3-containing aqueous solution at 47° C. and a flow rate of 2.8 cm / s for 5.0 hours, thereby producing goethite particles. Meanwhile, it was confirmed that the pH value of the aqueous solution during the air passage was maintained at 8.5 to 9.5.
[0213]The water suspension containing the thus obtained goethite par...
examples 14 to 17
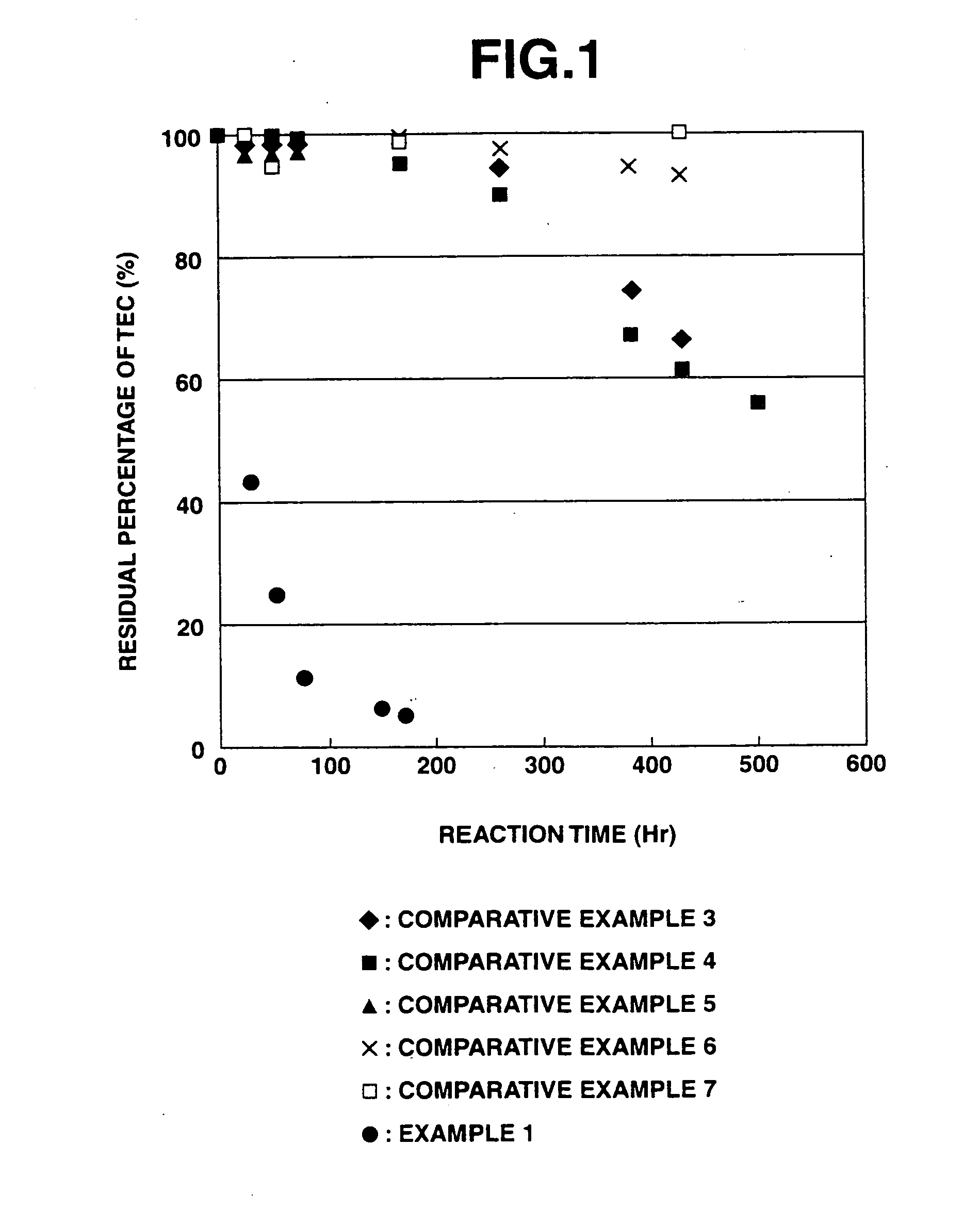
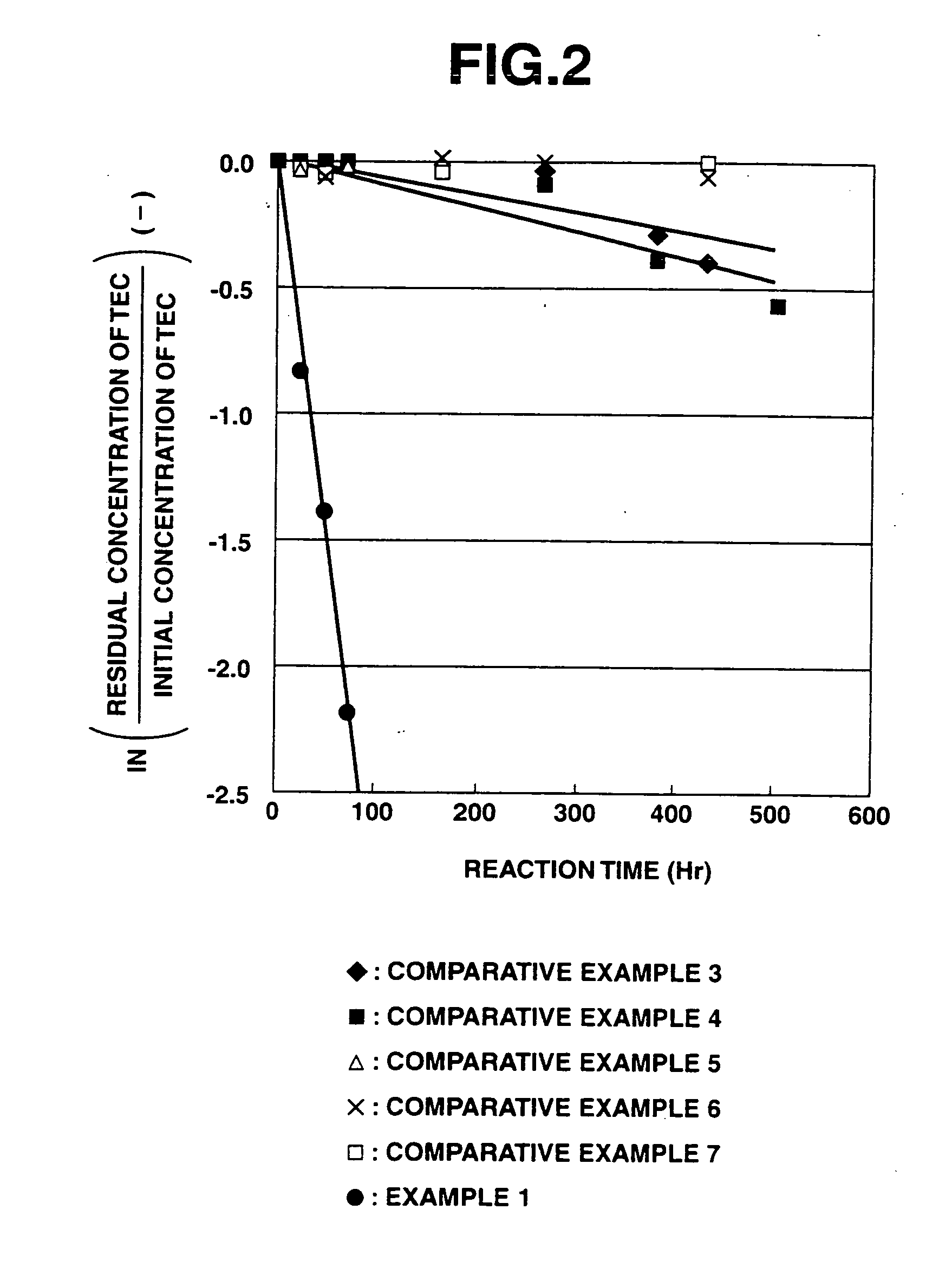
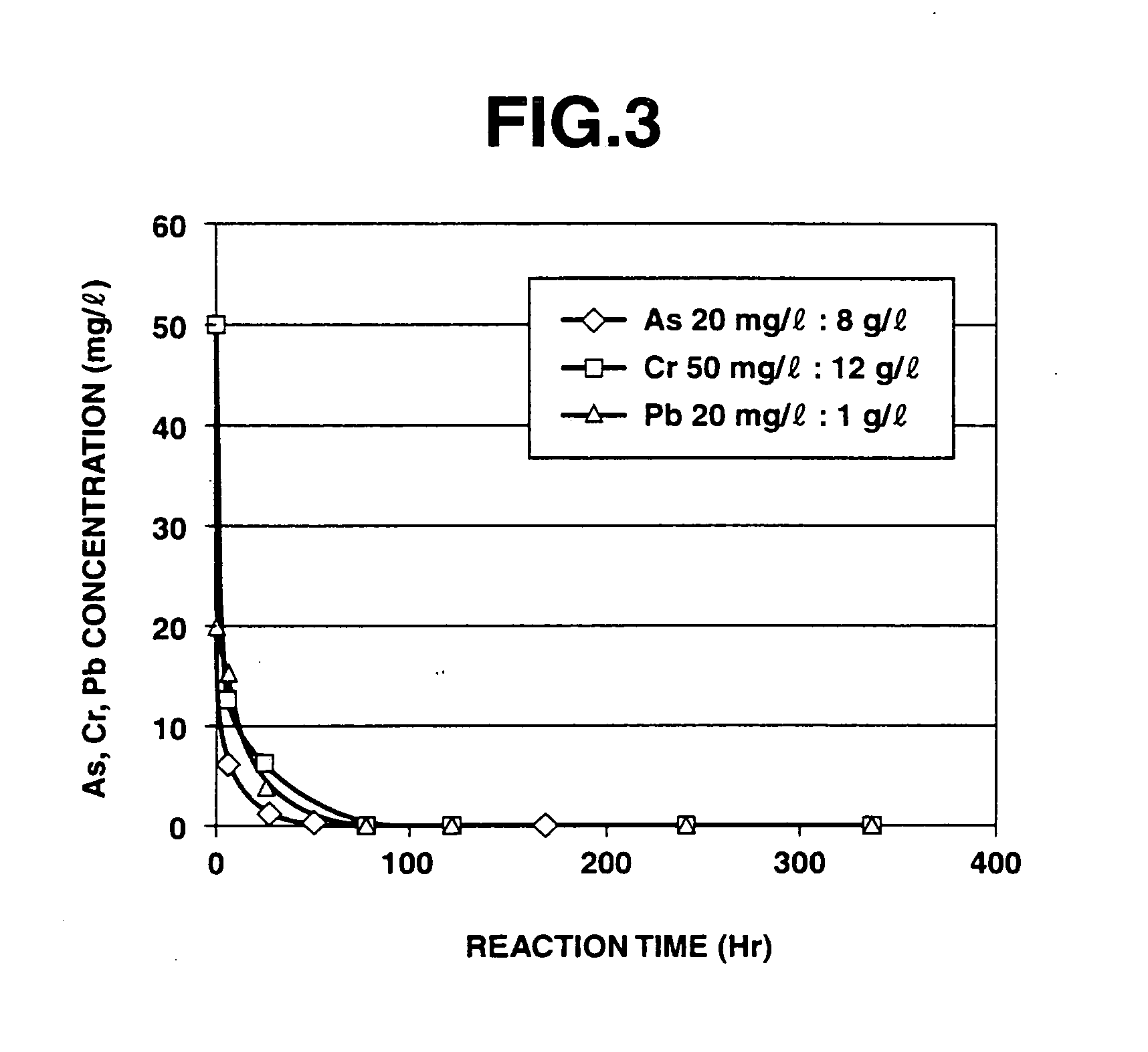
Results of Evaluation of Insolubilization Reaction of Heavy Metals (Apparent Reaction Rate Constant)
[0228]According to the above evaluation method, it was confirmed that when the purifying agent was used in the purification treatment of heavy metals, the apparent reaction rate constant for arsenic was 0.0195 h−1, the apparent reaction rate constant for chromium was 0.0138 h−1, and the apparent reaction rate constant for lead was 0.0630 h−1.
[0229]At this time, the solution used for measuring the residual amounts of heavy metals was maintained at a pH value of about 10 when adding any of arsenic, chromium and lead thereto, and the iron composite particles separated therefrom still exhibited a black color without any change. Therefore, it was suggested that the a ferrite compound combined with the heavy metals was formed.
[0230]On the contrary, in the case of the reduced iron and electrolytic iron used in the below-mentioned Comparative Examples, the solution used for measuring the resi...
examples 17 to 20
[0245]Various properties of the purifying iron composite particles and the purifying agent after preservation are shown in Table 8. Meanwhile, in Examples 17 to 20, the purifying agent obtained in Example 2 which had a solid content of 30% was preserved in an open system for a period of 1, 3, 6 and 12 months, respectively.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com