B-cyclodextrin derivatives and their use against anthrax lethal toxin
a technology of b-cyclodextrin and anthrax, which is applied in the direction of antibacterial agents, antinoxious agents, drug compositions, etc., can solve the problems of reducing ineffective and less attractive antibodies as potential drugs, so as to reduce the effectiveness of antibiotic treatment and effective treatment of inhalational anthrax
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of β-cyclodextrin Derivatives
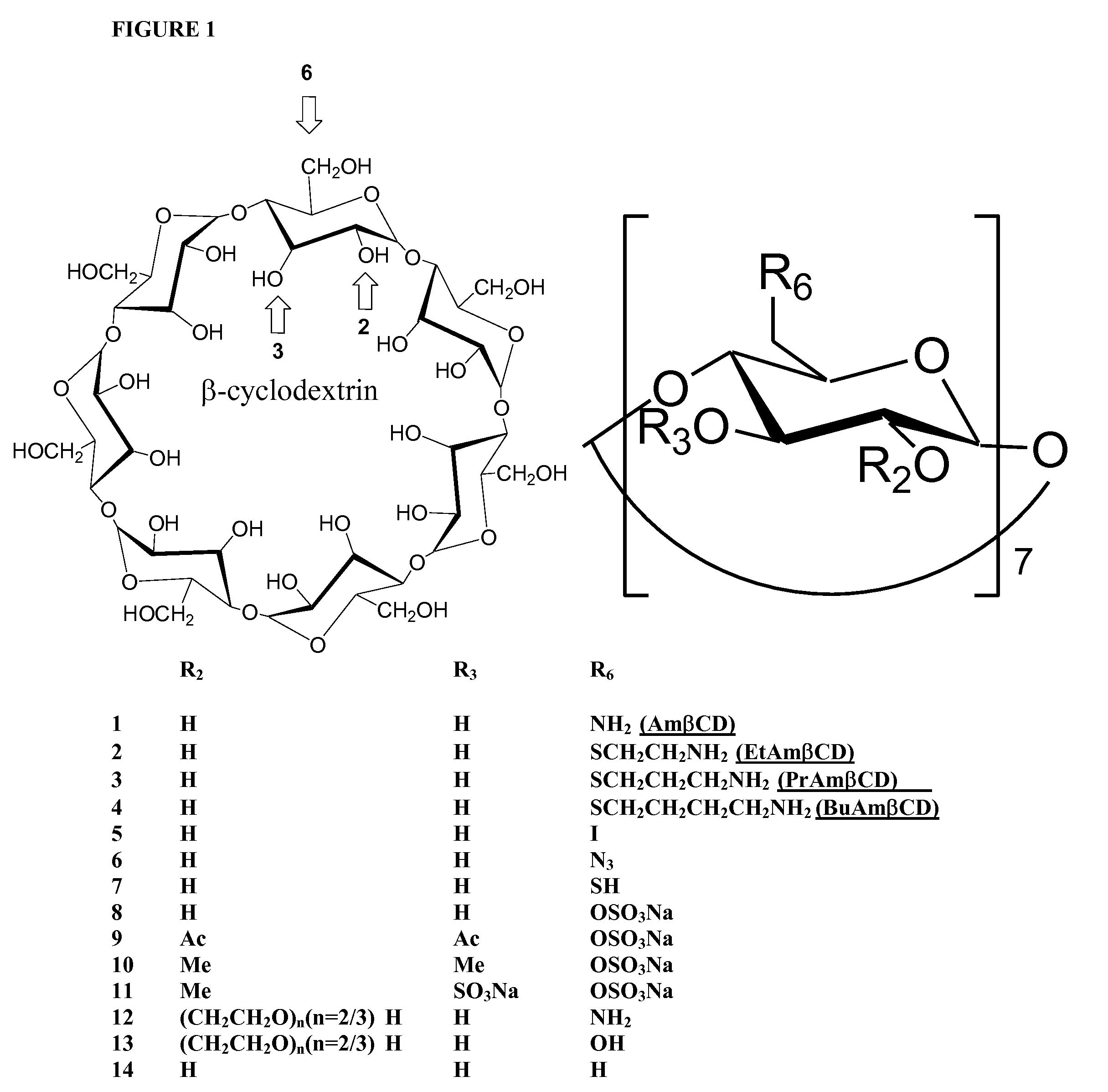
[0034]Reagents. β-cyclodextrin derivatives 1-7 listed in Table 1 were synthesized at Pinnacle Pharmaceuticals, Inc. (Charlottesville, Va.). Compounds 12 and 13 were purchased from Cytrea Ltd (Dublin, Ireland). Sulfo derivatives of β-cyclodextrin 8-11 were kindly provided by Dr. Gyula Vigh (Texas A&M University, College Station, Tex.). β-cyclodextrin 14 was purchased from Sigma (St. Louis, Mo.). Most chemical reagents were purchased from Aldrich Chemicals or Fisher Scientific and used without further purification. Acetonitrile and dichloromethane were distilled from CaH2. DMF was distilled from CaH2 under diminished pressure. Triethylamine was distilled from P205.
[0035]Analysis. 1H NMR and 13C NMR spectra were recorded on a General Electric QE-300 or a Varian 300 spectrometer. Moisture sensitive reactions were conducted under argon in oven-dried glassware. Analytical thin-layer chromatography was performed on Merk 60F254 precoated silica gel pla...
example 2
Protection of Cells from Cytotoxicity
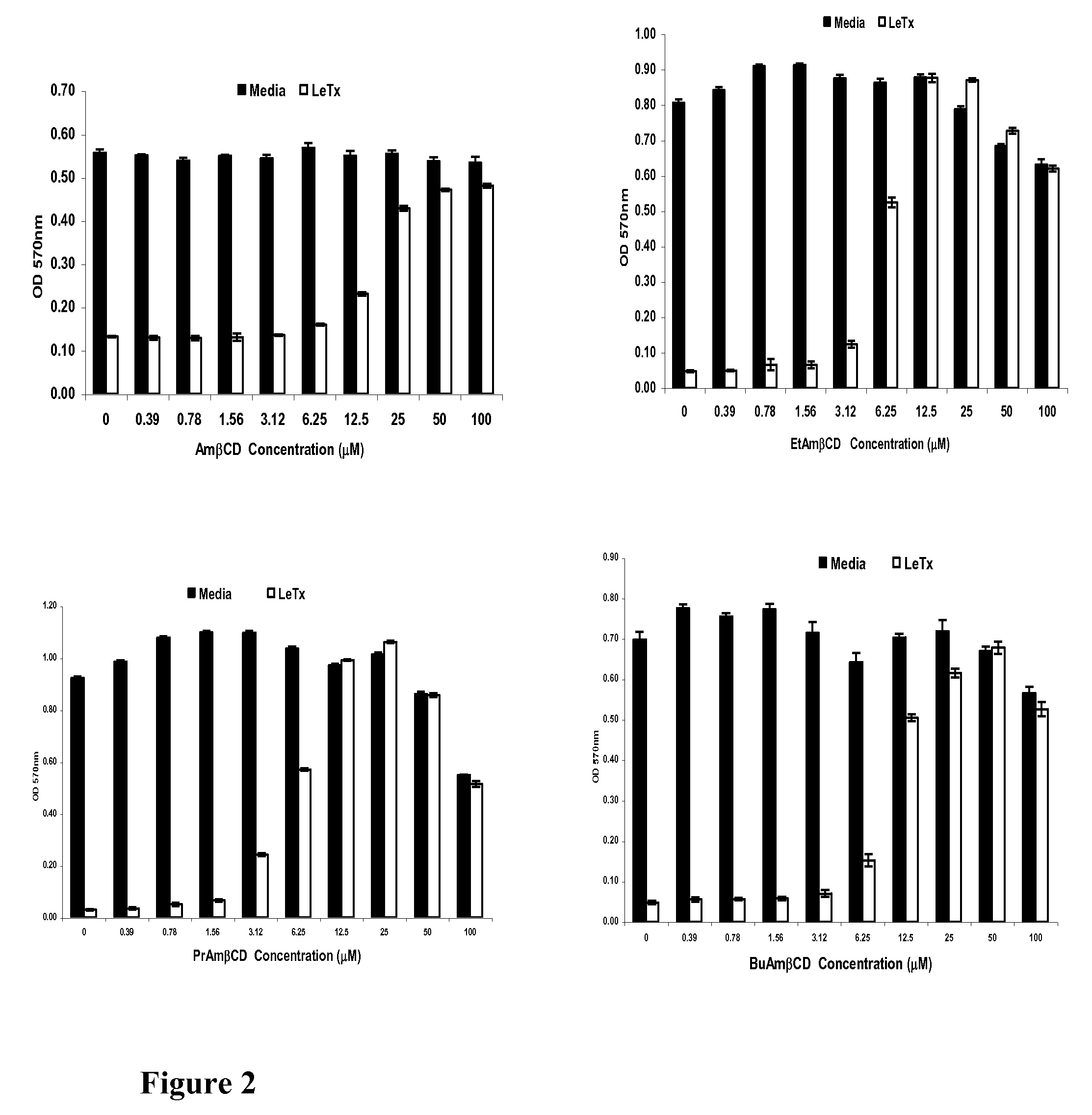
[0036]Recombinant B. anthracis lethal factor (rLF), edema factor (rEF), and protective antigen (rPA) were acquired from List Biological Laboratories, Inc. (Campbell, Calif.). Murine RAW 264.7 monocyte-macrophage cell line ATCC TIB-71 was obtained from American Type Culture Collection (Manassas, Va., USA). The cells were cultured in phenol free Dulbecco's Modification of Eagle's Medium / Ham's F-12 50 / 50 Mix (Mediatech, Inc., Herndon, Va., USA) supplemented with 10% heat-inactivated fetal bovine serum, 100 units / ml: 100 μg / ml penicillin-streptomycin, 0.1 mM non-essential amino acids, and 0.5 mM 2-mercaptoethanol at 37° C. in 5% CO2. The cells were harvested using Cellstripper™ from Mediatech, Inc. and then were washed once with media to remove the non-enzymatic dissociation solution. RAW 264.7 cells were plated in 96-well flat-bottomed tissue culture plates from Becton Dickinson (San Jose, Calif., USA) at a concentration of 105 cells / well in the DME...
example 3
Inhibition of Ion Conductance
[0037]Ion conductance experiments were performed according to Montal and Mueller [14] with modifications [15,16]. PA channels were reconstituted into planar lipid membranes formed from DPhPC; the membrane bathing solution contained 0.1M KCl, 1 mM EDTA at pH 6.6. Ion conductance through PA channels was measured in the presence of PrAmBC.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hydrophobic | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com