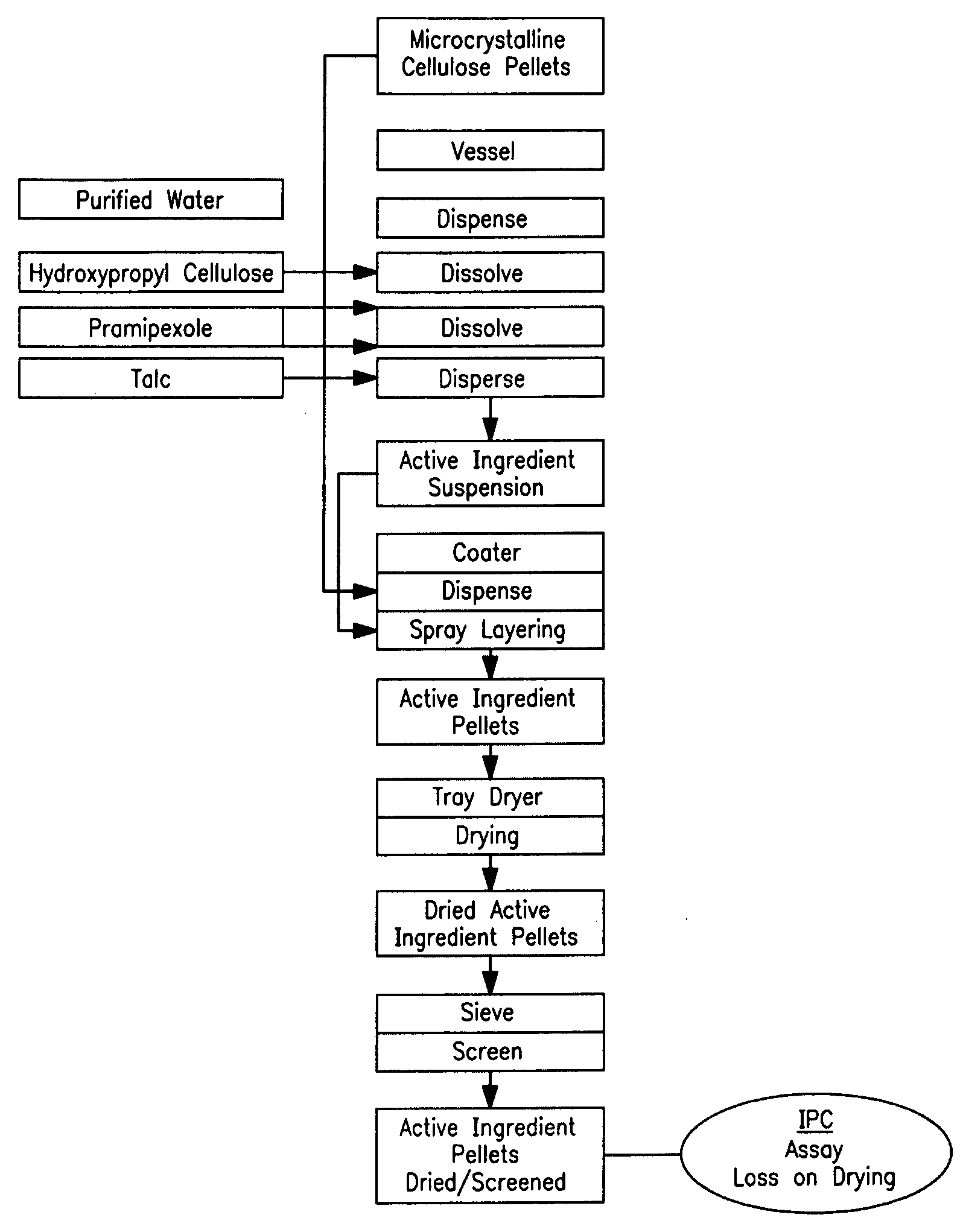
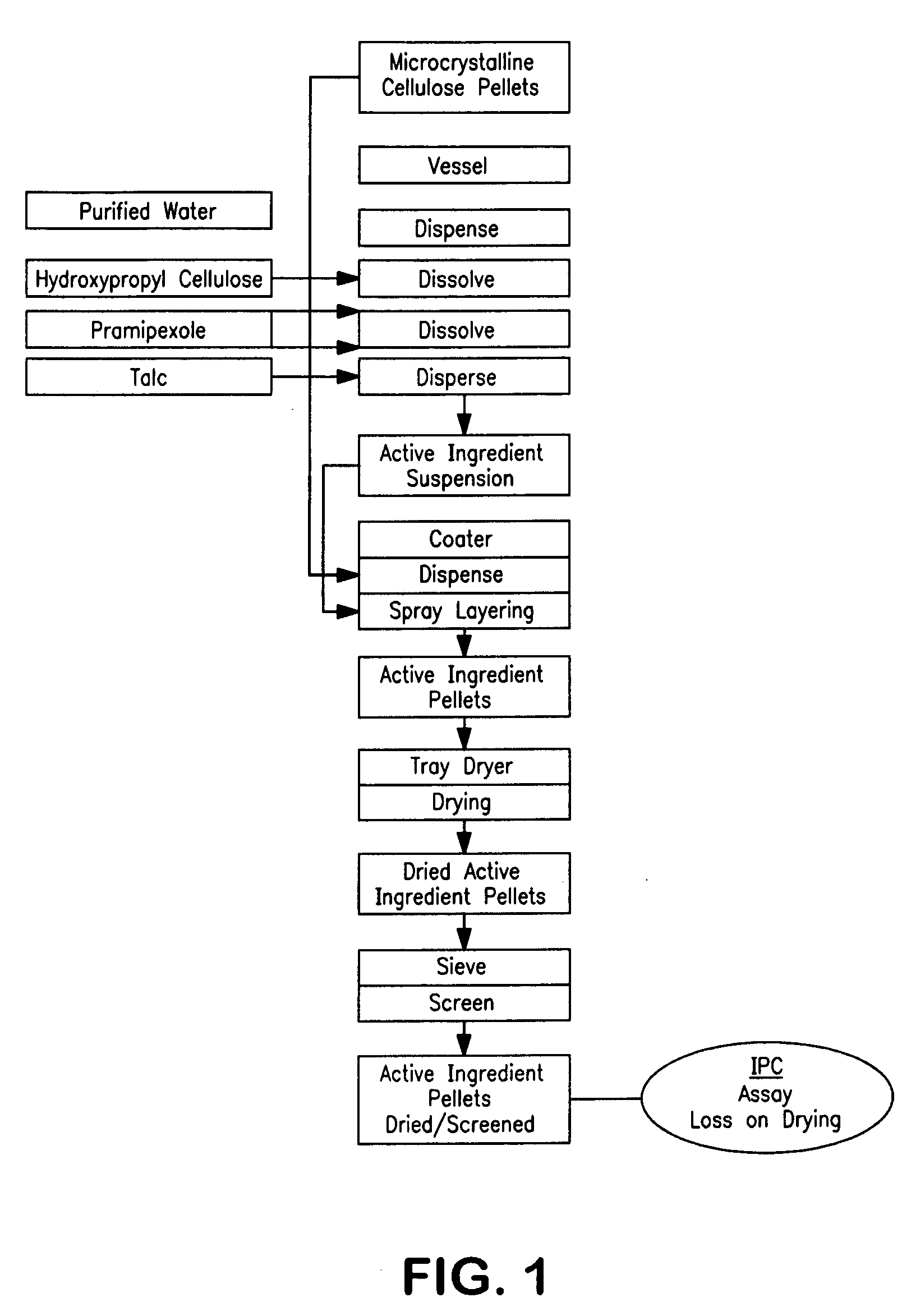
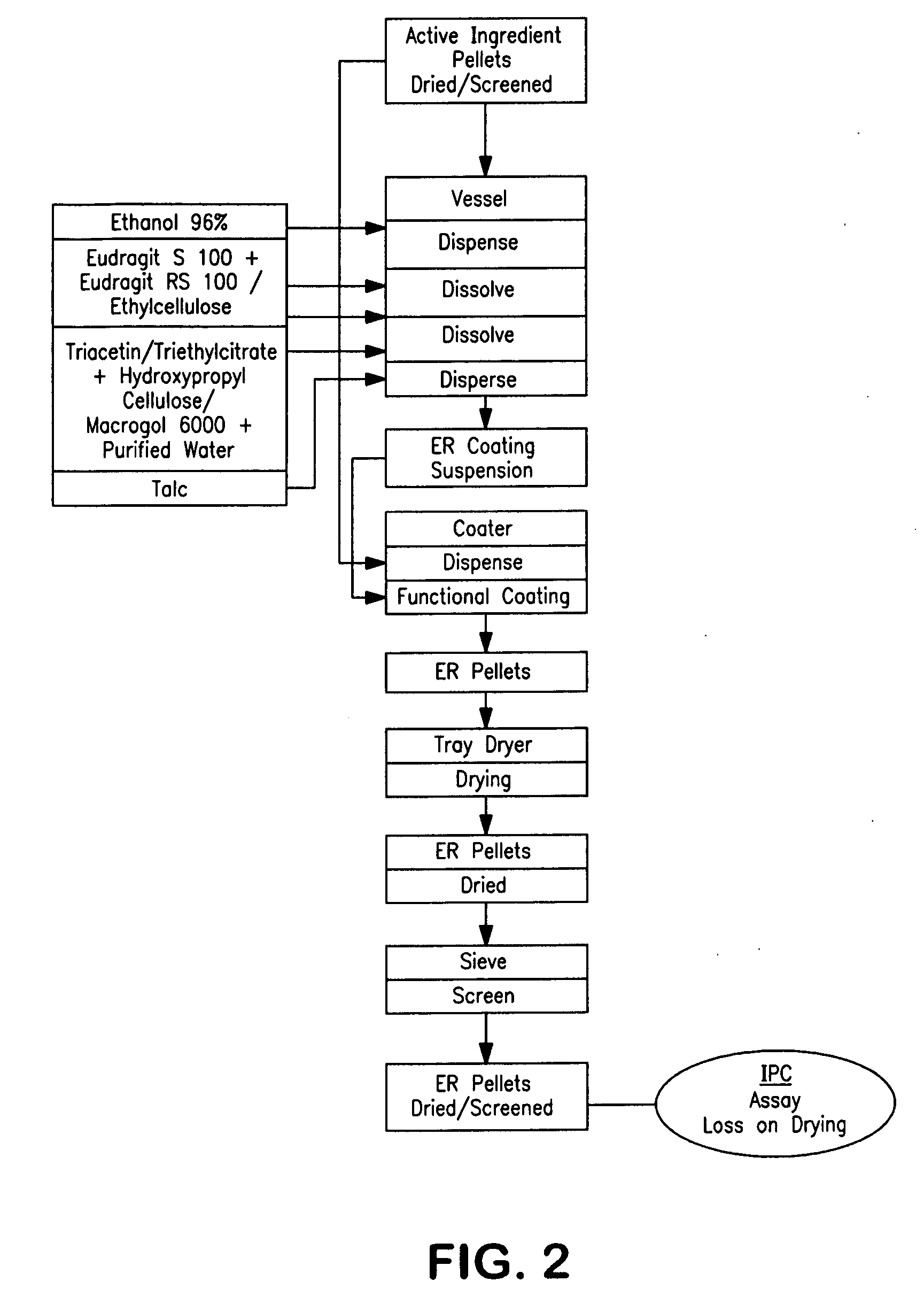
Extended release pellet formulation containing pramipexole or a pharmaceutically acceptable salt
a technology of extended release and pellet formulation, which is applied in the direction of biocide, microcapsules, drug compositions, etc., can solve the problems of difficult formulation of dosage forms having a suitable combination of modified, extended, sustained,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0134]One embodiment of the qualitative and quantitative composition of pramipexole extended release pellets according to the present invention (Formulation D) is shown in Table 1.
TABLE 1Qualitative and Quantitative Composition ofPramipexole Extended Release (ER) Capsule (Formulation D)mg permg per0.75 mg0.75 mgReference toIngredientcapsulecapsuleFunctionStandardsER Pellets consisting of:88.458Pramipexole dihydrochloride0.750Active ingredientCompanymonohydratestandardMicrocrystalline cellulose pellets73.980Non-pareille carrierPh.Eur. / NF(Cellets 700)pelletHydroxypropyl cellulose0.150Wet binderPh.Eur. / NF(KLUCEL ® EF)Talc0.495GlidantPh.Eur. / USPMethacrylic acid copolymer,7.500Functional coatingPh.Eur. / NFType B (EUDRAGIT ® S 100)Ammonio methacrylate3.750Functional coatingPh.Eur. / NFcopolymer, Type B(EUDRAGIT ® RS 100)Triacetin1.833PlasticizerPh.Eur. / USPEthanol (96%)173.333*SolventPh.Eur.Purified water30.000*SolventPh.Eur. / USPHPMC capsule, size 346.000ShellCompanyStandardTotal134.45888.458...
example 2
[0135]One embodiment of the qualitative and quantitative composition of pramipexole extended release pellets according to the present invention Formulation B) is shown in Table 2.
TABLE 2Qualitative and Quantitative Composition ofPramipexole ER Capsule (Formulation E)mg permg per0.75 mg0.75 mgReference toIngredientcapsulecapsuleFunctionStandardsER Pellets consisting of:91.600Pramipexole dihydrochloride0.750Active ingredientCorporatemonohydratestandardMicrocrystalline cellulose pellets73.980Non-pareille carrierPh.Eur / NF(Cellets 700)pelletHydroxypropyl cellulose0.150Wet binderPh.Eur. / NF(KLUCEL ® EF)Talc0.578GlidantPh.Eur. / USPMethacrylic acid copolymer,9.250Functional coatingPh.Eur. / NFType B (EUDRAGIT ® S 100)Ammonio methacrylate4.625Functional coatingPh.Eur. / NFcopolymer, Type B(EUDRAGIT ® RS 100)Triacetin2.267PlasticizerPh.Eur. / USPEthanol (96%)214.167*SolventPh.Eur.Purified water30.000*SolventPh.Eur. / USPHPMC capsule, size 346.000ShellCompanyStandardTotal137.60091.600*removed during pro...
example 3
[0136]One embodiment of the qualitative and quantitative composition of pramipexole extended release pellets according to the present invention (Formulation F) is shown in Table 3.
TABLE 3Qualitative and Quantitative Composition ofPramipexole ER Capsule (Formulation F)mg permg per0.75 mg0.75 mgReference toIngredientcapsulecapsuleFunctionStandardsER Pellets consisting of:80.063Pramipexole dihydrochloride0.750Active ingredientCorporatemonohydratestandardMicrocrystalline cellulose pellets73.980Non-pareille carrierPh.Eur / NF(Cellets 700)pelletHydroxypropyl cellulose0.150Wet binderPh.Eur. / NF(KLUCEL ® EF)Talc0.495GlidantPh.Eur. / USPEthyl cellulose (N14)3.750Functional coatingPh.Eur. / NFMacrogol 60000.938PlasticizerPh.Eur. / USPEthanol (96%)49.167*SolventPh.Eur.Purified water32.583*SolventPh.Eur. / USPHPMC capsule, size 346.000ShellCompanyStandardTotal126.06380.063*removed during processing (does not appear in the final product)
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| solubility | aaaaa | aaaaa |
| solubility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com