Activin receptor-like kinase-1 compositions and methods of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Generation of an ALK1.Fc Molecule
[0400]Using standard molecular biology techniques, an ALK1.Fc molecule was generated by attaching the extracellular domain of ALK-1 (amino acid residues 1-118 of human ALK-1) to the Fc region of human IgG1 via a polypeptide linker. Briefly, an extracellular fragment of human ALK-1 (amino acid 1-118) was subcloned into a pRK5 vector that had been engineered for the expression of fusion protein with a C-terminal Fc of human IgG1. ALK1.Fc was purified with Protein-A affinity chromatography from conditioned medium harvested from serum-free culture of CHO cells transiently transfected with the expression plasmid. The ALK1.Fc molecule had the following cDNA and amino acid sequences:
ALK1.Fc cDNA sequence:(SEQ ID NO: 1)ATGACCTTGGGCTCCCCCAGGAAAGGCCTTCTGATGCTGCTGATGGCCTTGGTGACCCAGGGAGACCCTGTGAAGCCGTCTCGGGGCCCGCTGGTGACCTGCACGTGTGAGAGCCCACATTGCAAGGGGCCTACCTGCCGGGGGGCCTGGTGCACAGTAGTGCTGGTGCGGGAGGAGGGGAGGCACCCCCAGGAACATCGGGGCTGCGGGAACTTGCACAGGGAGCTCTGCAGGGGGCGCCCC...
example 2
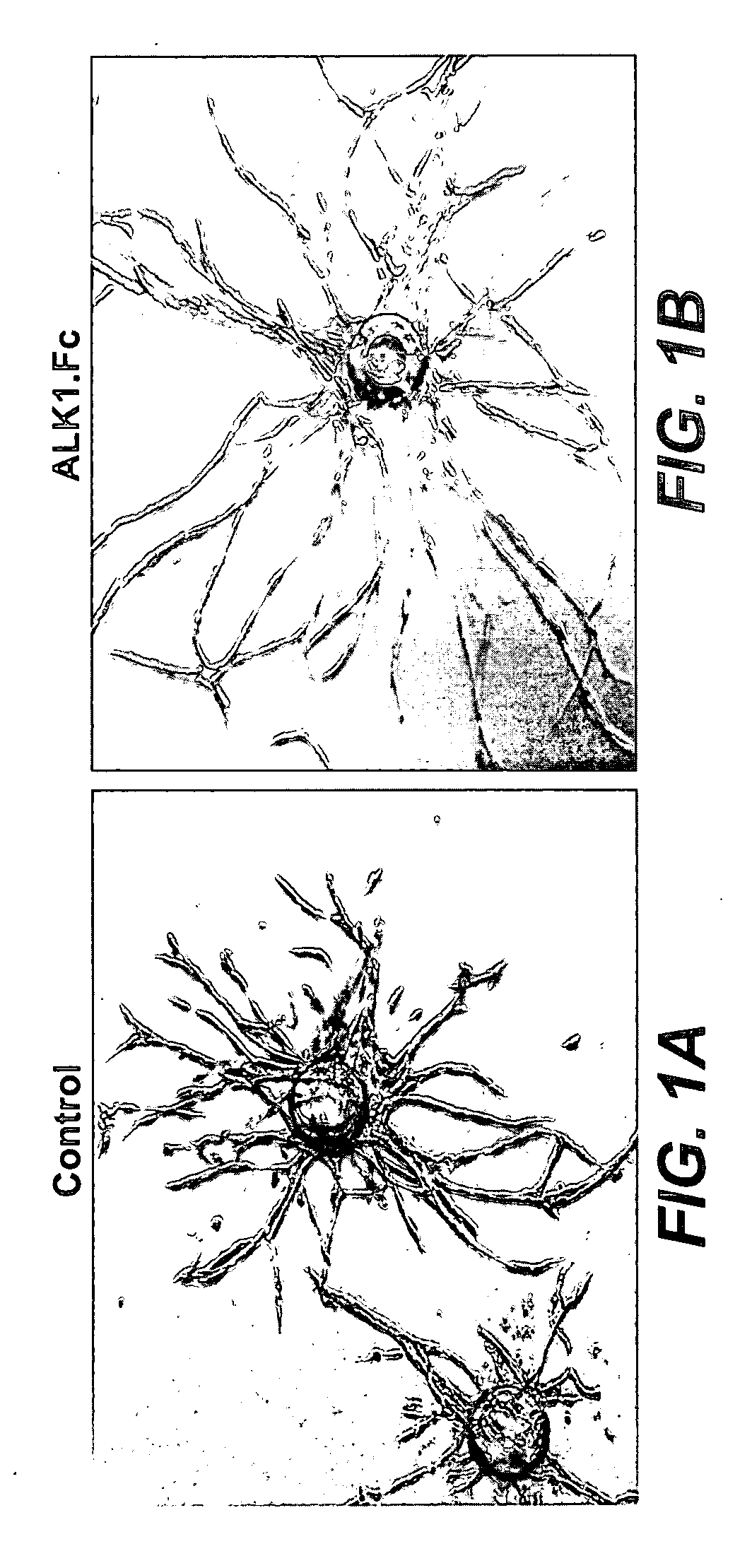
Treatment with ALK1.Fc Enhances Endothelial Cell Sprouting In Vitro
[0401]The effect of inhibiting ALK-1 signalling on endothelial cell growth was explored using the HUVEC fibrin gel bead assay. Details of the HUVEC fibrin gel bead assay have been previously described (Nakatsu, Minn., Microvascular Research 44:102-112 (2003)). Briefly, Cytodex 3 beads (Amersham Pharmacia Biotech) were coated with 350-400 human umbilical vein endothelia cells (HUVECs) per bead in 2 ml EGM-2 medium (Clonetics). About 200 LUVECs-coated beads were imbedded in fibrin clot in one well of 12-well tissue culture plate. 80×103 D551 human fibroblast cells were plated on top of the clot and medium was changed every 2 days. ALK1.Fc was added to the culture medium at 5 ug / ml. Phase-contrast microscopy images were taken on day 9.
[0402]HUVECs growing in fibrin gels in the presence of co-cultured human fibroblast cells generate sprouts with a distinct lumen-like structure (Nakatsu, M. N. et al. Microvasc Res 66, 102...
example 3
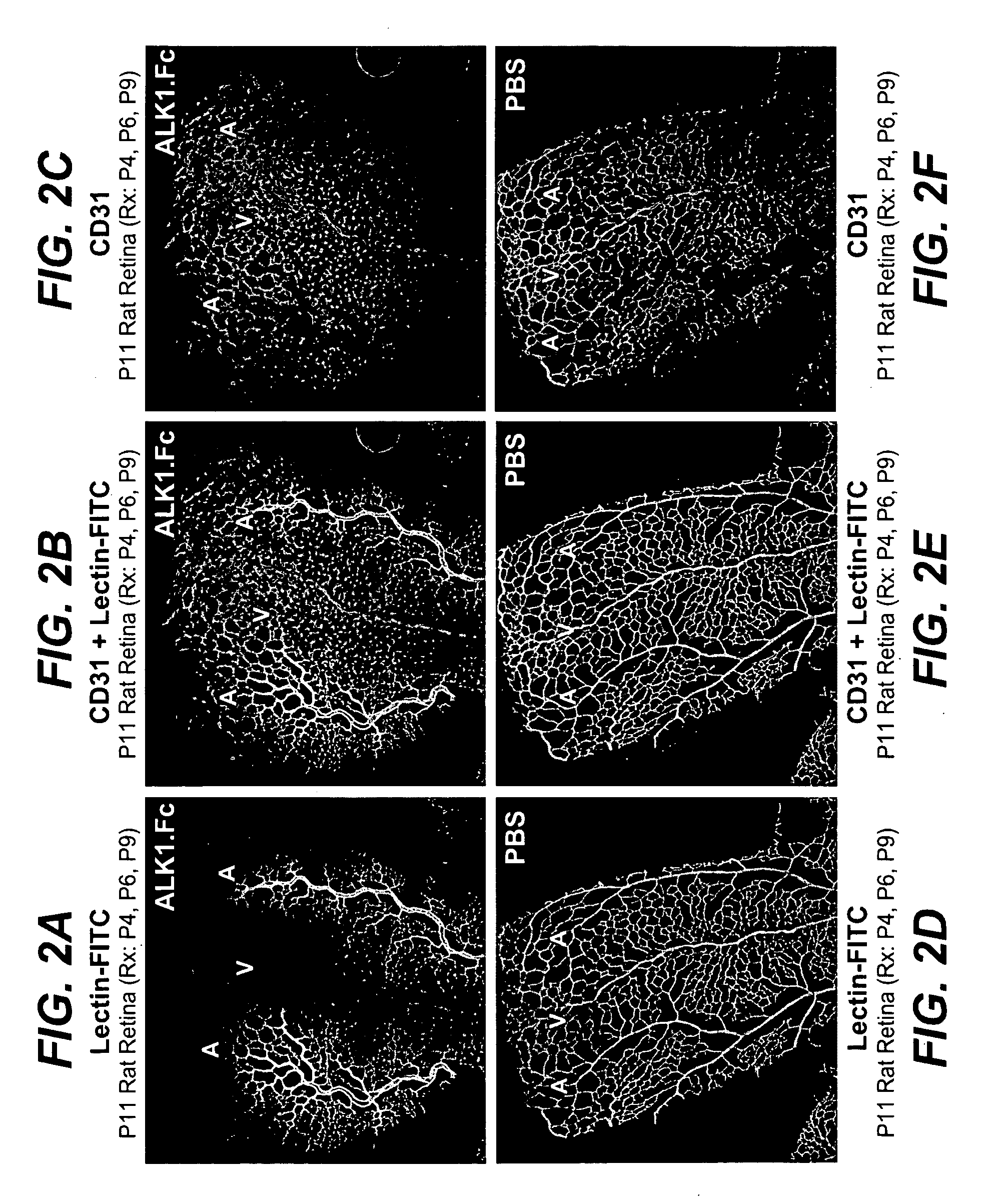
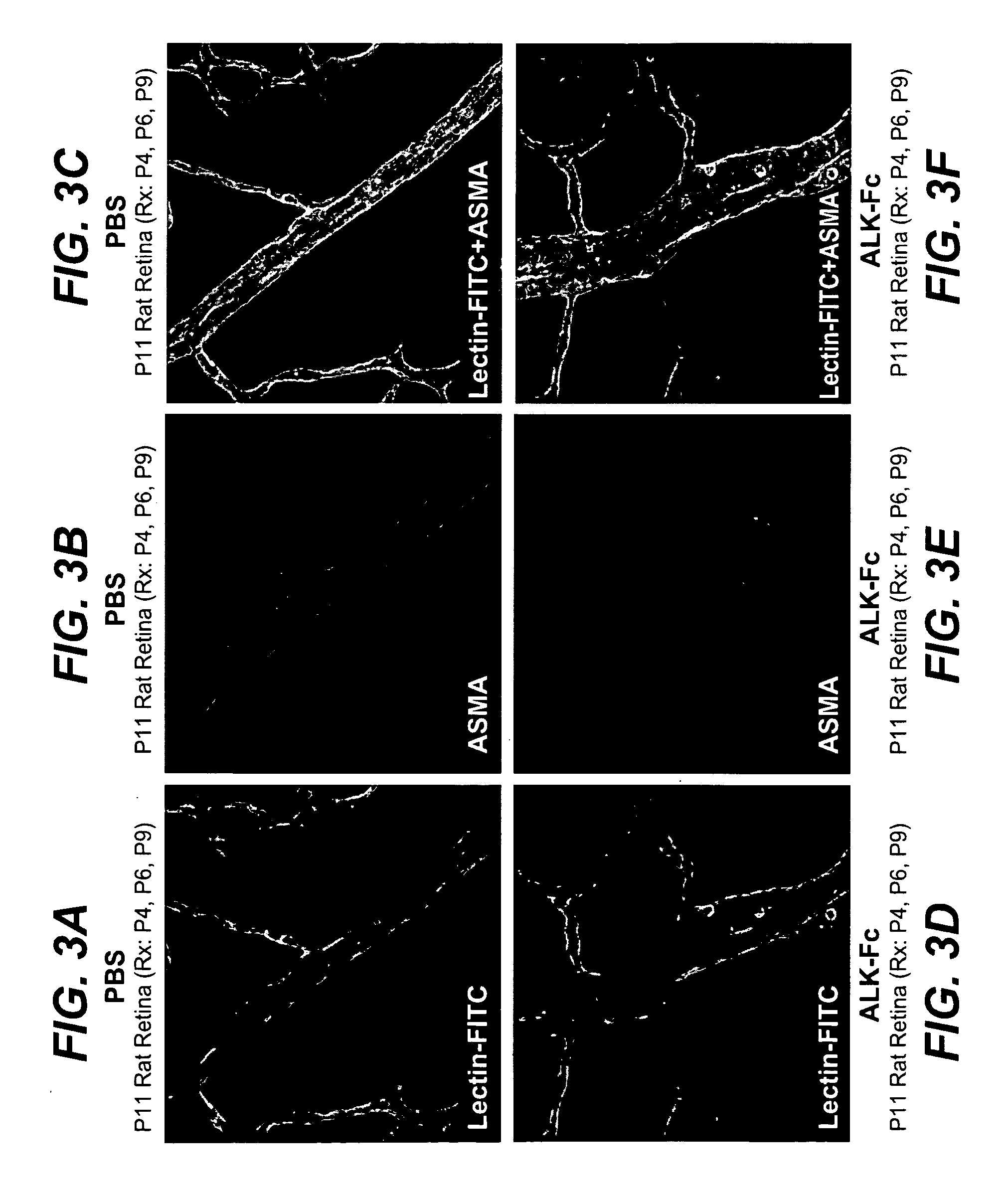
Treatment with ALK1-Fc Affects Retinal Vascular Development In Vivo
[0403]Neonatal SD rats from the same litters were used to study the effect of ALK1.Fc on retinal vascular development. Rats were injected i.p. with ALK1.Fc (10 mg / kg body weight) on postnatal day 4 (P4), P6 and P9. On P11, rats were anesthetized with Isoflurane. FITC-labeled Lycopersicon Esculentum Lectin (150 μg in 150 μl of 0.9% NaCl; Vector Laboratories) was injected intracardially and allowed to circulate for 3 min. Eyes were collected and fixed with 4% PFA in PBS overnight, followed by PBS washes. The dissected retinas were blocked with 10% goat serum in PBST (PBS, 0.5% Triton X-100) for 3 hrs, then incubated overnight with biotinylated isolectin B4 (1:100, Bandeiraea simplicifolia; Molecular Probe) or Cy3-conjugated anti-alpha smooth muscle actin (ASMA, 1:200, Sigma-Aldrich), with 10% goat serum in PBST. To visualize the biotynylated isolectin B4, retinas were then washed 4 times in PBST, and incubated overnigh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Inhibition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com