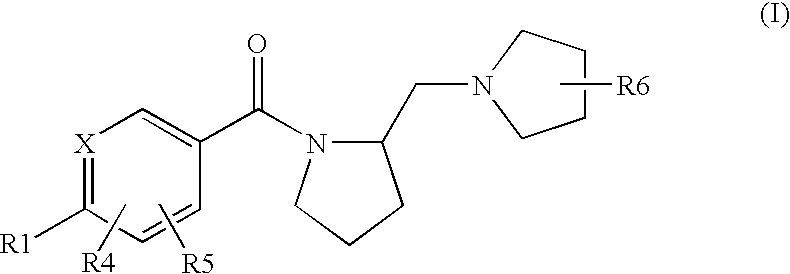
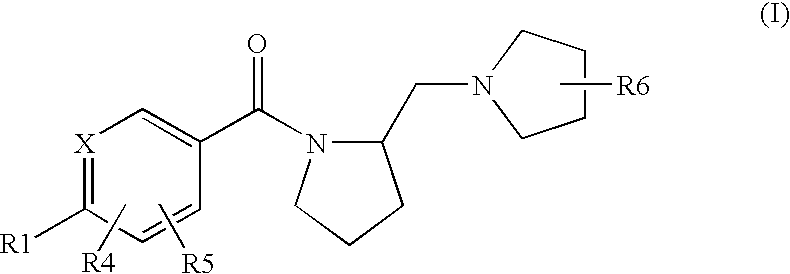
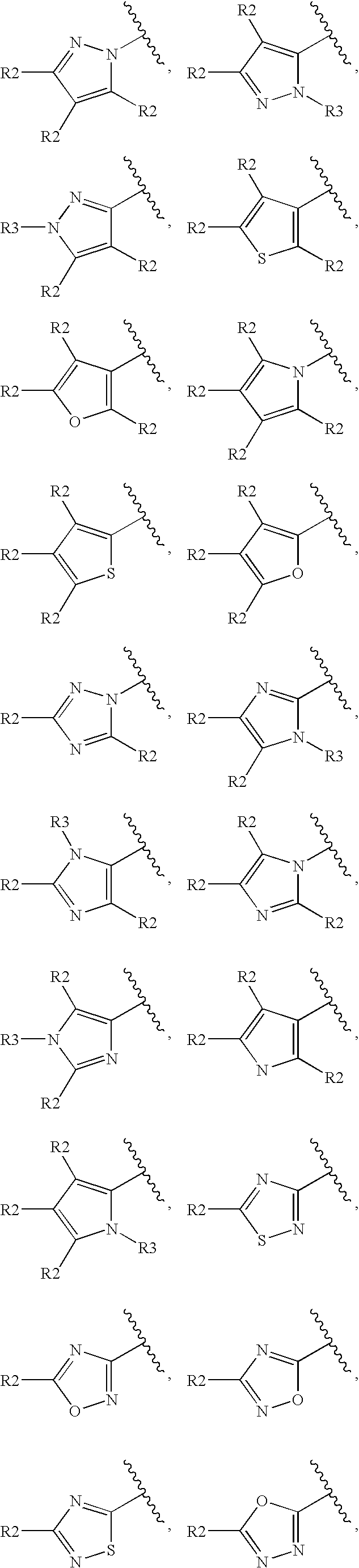
Histamine H3 Receptor Agents, Preparation and Therapeutic Uses
a technology of histamine h3 receptor and histamine, applied in the field of new drugs, can solve the problems of poor blood-brain barrier penetration of imidazole-containing compounds, unsatisfactory side effects, and hepatic and ocular toxicities, and achieve selective and high affinity binding.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
internediate preparation 1
2.(R)-Methyl-1-(2-(S)-pyrrolidinylmethyl)pyrrolidine
[0143]
[0144](S) BOC proline (CAS 15761-39-4) and 2-(R)Methyl-pyrrolidine hydrochloride (CAS 135324-85-5) are coupled in a manner substantially analogous to General Procedure D in dichloromethane to give 2(S)-(2(R)-Methyl-pyrrolidine-1-carbonyl)pyrrolidine-1-carboxylic acid tert-butyl ester(233179). The material is deprotected by stirring in dichloromethane at 5-10° C. while trifluoroacetic acid (10 eq,) is added and then stirred at room temperature for 18 hours. Reaction is concentrated and dissolved in H2O. The pH is adjusted to 8-9 with K2CO3, and the aqueous layer is extracted several times with CH2Cl2. The extracts are combined, dried (Na2SO4) and concentrated in vacuo to give (2(R)-Methyl-pyrrolidin-1-yl)-pyrrolidin-2-yl-methanone. A 1 M Lithium Aluminum Hydride / THF solution (3 eq.) is diluted with an equal volume of THF and stirred under N2. A THF solution of (2(R)-methyl-pyrrolidin-1-yl)-pyrrolidin-2-yl-methanone is added dr...
example 1
S-[4-(2(S)-Pyrrolidin-1-ylmethyl-pyrrolidine-1-carbonyl)-phenyl)-thiophene-2-carbonitrile
[0209]
[0210]The title compound is prepared in a manner substantially analogous to General Procedure D in 100 mL 10% DMF / dichloromethane using 4(5-cyano-thiophen-2-yl)-benzoic acid (CAS 402765-55-9)(2.75 g, 12.0 mmol), EDC—HCl (3.44 g, 18.0 mmol), HOBt (2.43 g, 18.0 mmol), DIEA (5.22 mL, 30 mmol) and (S)(+)-1-(2-pyrrolidinylmethyl)pyrrolidine (1.54 g, 10.0 mmol) to give the title compound (2.33 g, 64% yield). MS (ES+) 366.2 (M+H)+
example 2
5-{4-[2-(2(S)-(2-(R)-methyl-pyrrolidin-1-ylmethyl)-pyrrolidine-1-carbonyl]-phenyl}-thiophene-2-carbonitrile
[0211]
[0212]The title compound is prepared in a manner substantially analogous to General Procedure D in 10 mL 10% DMF / dichloromethane using 4-(5-cyano-thiophen-2-yl)-benzoic acid (CAS 402765-55-9)(360 mg, 1.57 mmol), EDC—HCl (451 mg, 2.36 mmol), HOBt (319 mg, 2.36 mmol), DIEA (1.14 mL, 6.5 mmol) and 2-(R)Methyl-1-(2-(S)-pyrrolidinylmethyl)pyrrolidine di HCl(314 mg, 1.31 mmol) to give the title compound (60 mg, 13% yield). MS (ES+) 380.3 (M+H)+
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com