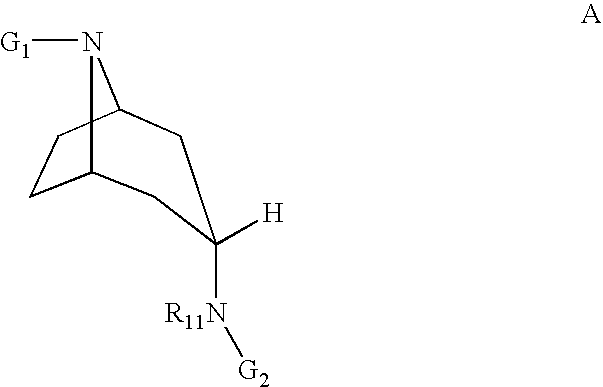
11 beta-hsd1 modulators
a technology of hydroxysteroid dehydrogenase and modulator, which is applied in the direction of biocide, drug composition, metabolic disorder, etc., can solve the problems of reducing central obesity, reducing food intake, and reducing expansion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1 (
Example 1(B)
tert-butyl 8-azabicyclo[3.2.1]octan-3-endo-ylcarbamate
[0588]
[0589] STEP 1: To a 5 L round-bottom flask was added 8-methyl-8-azabicyclo[3.2.1]octan-3-endo-amine (432 g, 3.1 mol), 2 L of dry 1,4-dioxane, 675 mL of deionized water and 468 g of dry triethylamine. Di-tert-butyl dicarbonate (solution in 1.2 L of dioxane) was added dropwise to the stirring solution at room temperature over 16 h. The reaction mixture was concentrated and the resulting residue suspended in 2.5 L of methylene chloride, then washed twice with 1 L of water, dried with anhydrous magnesium sulfate, filtered, and volatile organics removed by rotary evaporation to yield 617 g (83%) of tert-butyl 8-methyl-8-azabicyclo[3.2.1]octan-3-ylcarbamate (mp 79-81° C.).
[0590] STEP 2: To a 5 L round-bottom flask was added 480 g (2.0 mol) of tert-butyl 8-methyl-8-azabicyclo[3.2.]octan-3-endo-ylcarbamate, 2 L of toluene, and 69 g (0.5 mol) of potassium carbonate. 2,2,2-Trichloroethyl chloroformate (347 mL, 2.4 mol) ...
example 2
6-[3-endo-({[1-(4-chlorophenyl)cyclopropyl]carbonyl}amino)-8-azabicyclo[3.2.1]oct-8-yl]pyridine-3-carboxylic acid,
[0592]
[0593] A suspension of methyl 6-(3-endo-(1-(4-chlorophenyl)-cyclopropanecarboxamido)-8-azabicyclo[3.2.1]octan-8-yl)nicotinate (0.30 g, 0.69 mmol) from Example 1 in methanol (5 mL) was charged with 3N NaOH (1.0 mL, 3.0 mmol) and then heated 2 h at 50° C. After cooling to ambient temperature, the reaction mixture was neutralized to pH 5 using 1N HCl and then extracted with DCM (3×25 mL). The combined extracts were dried (anhyd Na2SO4) and concentrated under reduced pressure to afford the title compound (0.29 g, quant) as a white solid. 1H NMR (400 MHz, CDCl3): δ 8.83 (d, J=2.4 Hz, 1H), 8.01 (dd, J=9.1, 2.4 Hz, 1H), 7.44-7.38 (m, 4H), 6.43 (d, J=9.1 Hz, 1H), 5.83 (d, J=7.9 Hz, 1H), 4.51 (br s, 2H), 4.04-3.98 (m, 1H), 2.19-2.10 (m, 2H), 2.02-1.96 (m, 2H), 1.63-1.52 (m, 4H), 1.39-1.31 (2H), 1.07-1.02 (2H); MS (EI): 426 (MH+).
example 3
6-[3-endo-({[1-(4-chlorophenyl)cyclopropyl]carbonyl}amino)-8-azabicyclo[3.2.1]oct-8-yl]-N-cyclopropylpyridine-3-carboxamide,
[0594]
[0595] To a suspension of 6-[3-endo-({[1-(4-chlorophenyl)cyclopropyl]carbonyl}amino)-8-azabicyclo[3.2.1]oct-8-yl]pyridine-3-carboxylic acid (0.29 g, 0.68 mmol) from Example 2 in DCM (6 mL) was added 1,1′-carbonyldiimidazole (0.12 g. 0.75 mmol) with stirring. After 20 minutes, the reaction mixture was charged with DMAP (10 mg) and cyclopropylamine (46 μL, 0.80 mmol). After stirring 12 h, the reaction mixture was concentrated under reduced pressure. The residue was purified by chromatography (silica, EtOAc / Hex, 50:50 to 100:0) to give the title compound (99 mg, 31%) as a white solid. 1H NMR (400 MHz, CDCl2): δ 8.47 (d, J=2.4 Hz, 1H), 7.84 (dd, J=8.7, 2.4 Hz, 1H), 7.43-7.37 (m, 4H), 6.43 (d, J=9.2 Hz, 1H), 6.04 (br s, 1H), 5.83 (d, J=6.8 Hz, 1H), 4.43 (br s, 2H), 4.00-3.94 (m, 1H), 2.89-2.82 (m, 1H), 2.16-2.08 (m, 2H), 2.01-1.95 (m, 2H), 1.62-1.58 (m, 4H), ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com