Method of nuclear reprogramming
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
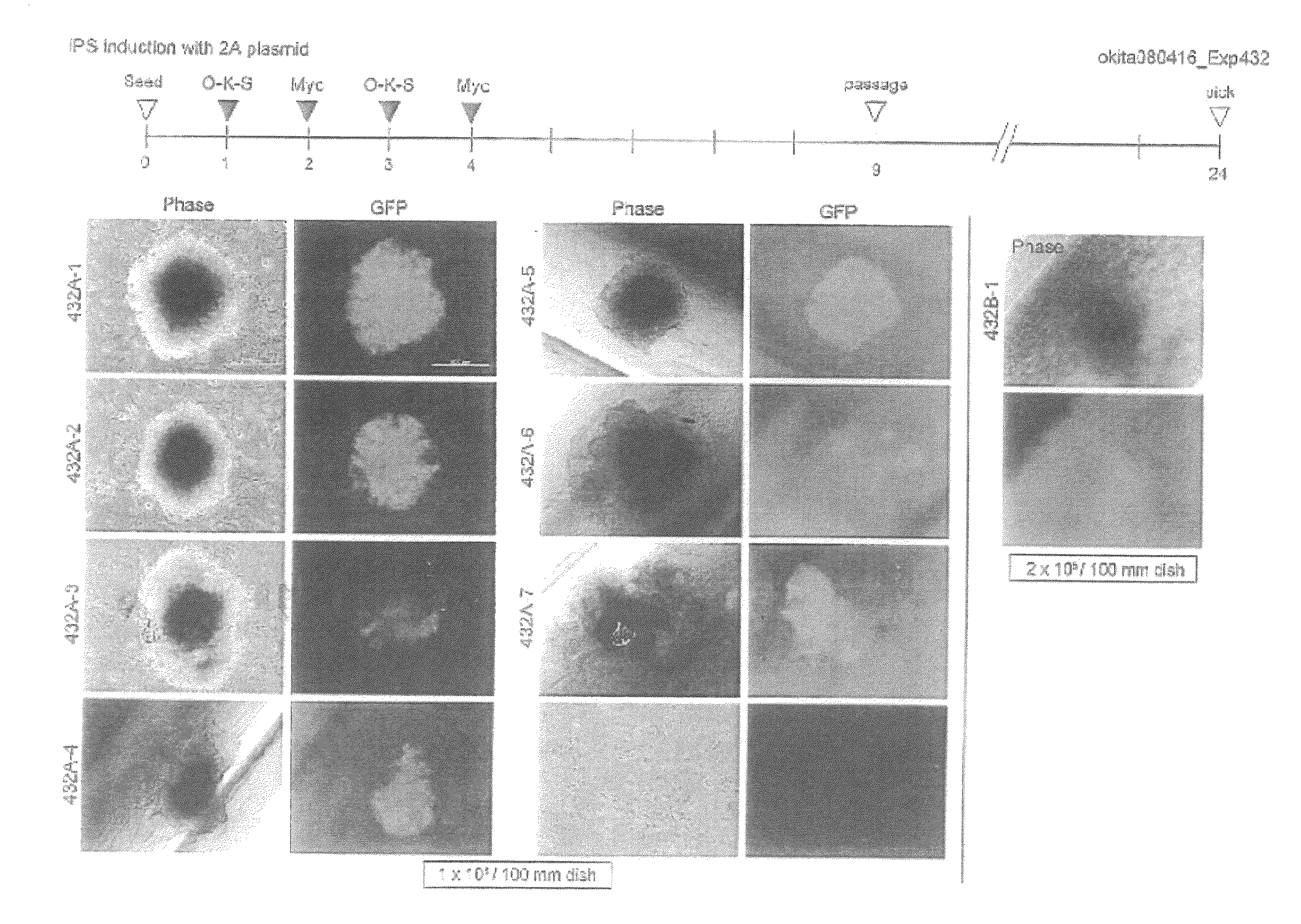
[0137]Mice having a Nanog reporter were used as an experimental system (Okita et al. Nature, Vol. 448, pp. 313-317, 2007). These mice were prepared by incorporating EGFP and a puromycin resistance gene into the Nanog gene locus of a BAC (bacterial artificial chromosome) purchased from BACPAC Resources. The mouse Nanog gene is expressed specifically in pluripotent cells such as ES cells and early embryos. Mouse iPS cells positive for this reporter have been shown to possess a differentiation potential nearly equivalent to that of ES cells. These Nanog reporter mice were mated with Fbx15 reporter mice (Tokuzawa et al. Mol Cell Biol, Vol. 23, 2699-2708 (2003)), whereby mutant mice having both the Nanog reporter and the Fbx15 reporter were generated.
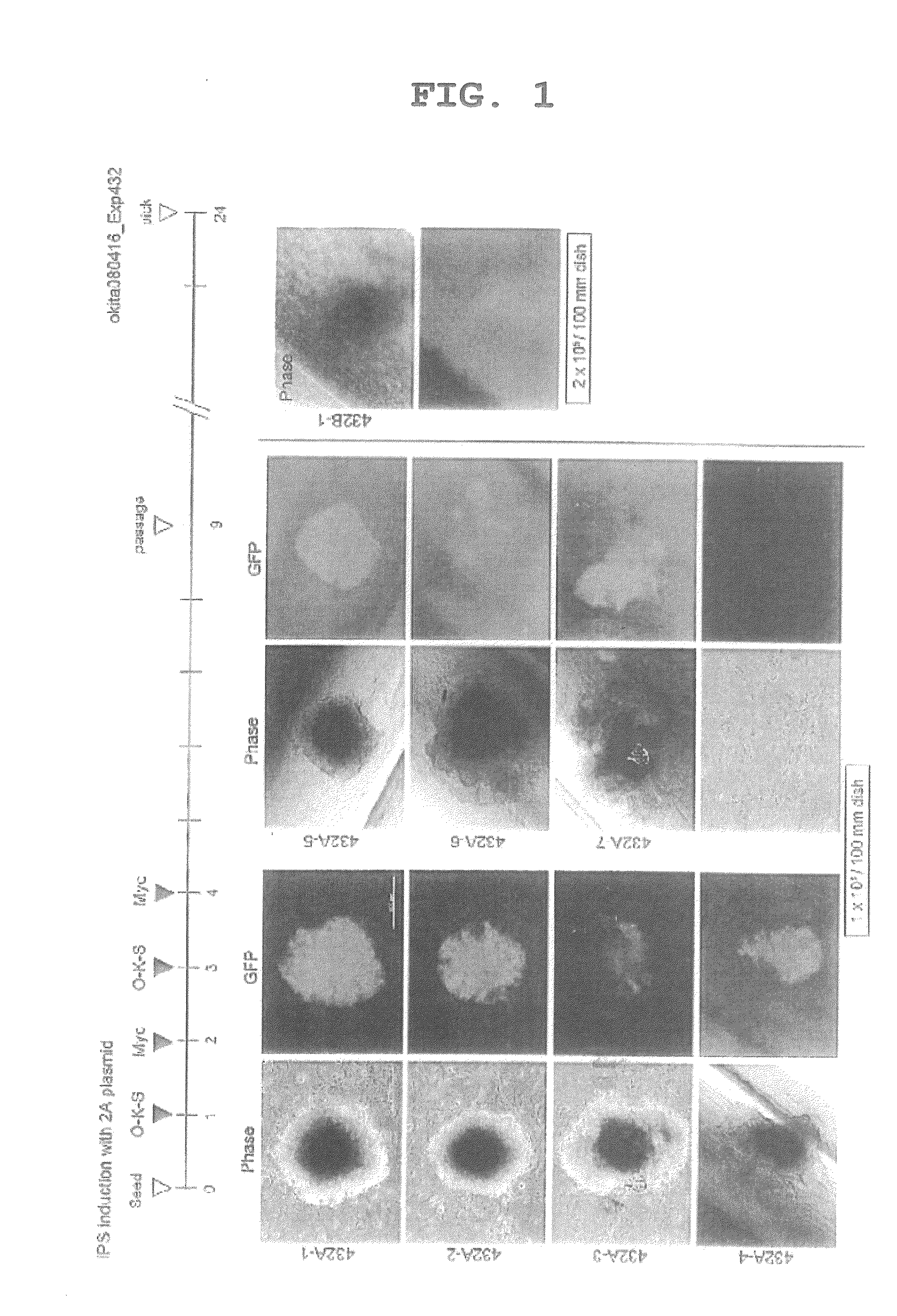
[0138]The plasmid used for reprogramming was prepared by treating pCX-EGFP (a plasmid supplied by Dr. Masaru Okabe at Osaka University: FEBS Letters, 407, 313-319, 1997) with EcoRI, and inserting a construct wherein the coding regions of Oct...
example 2
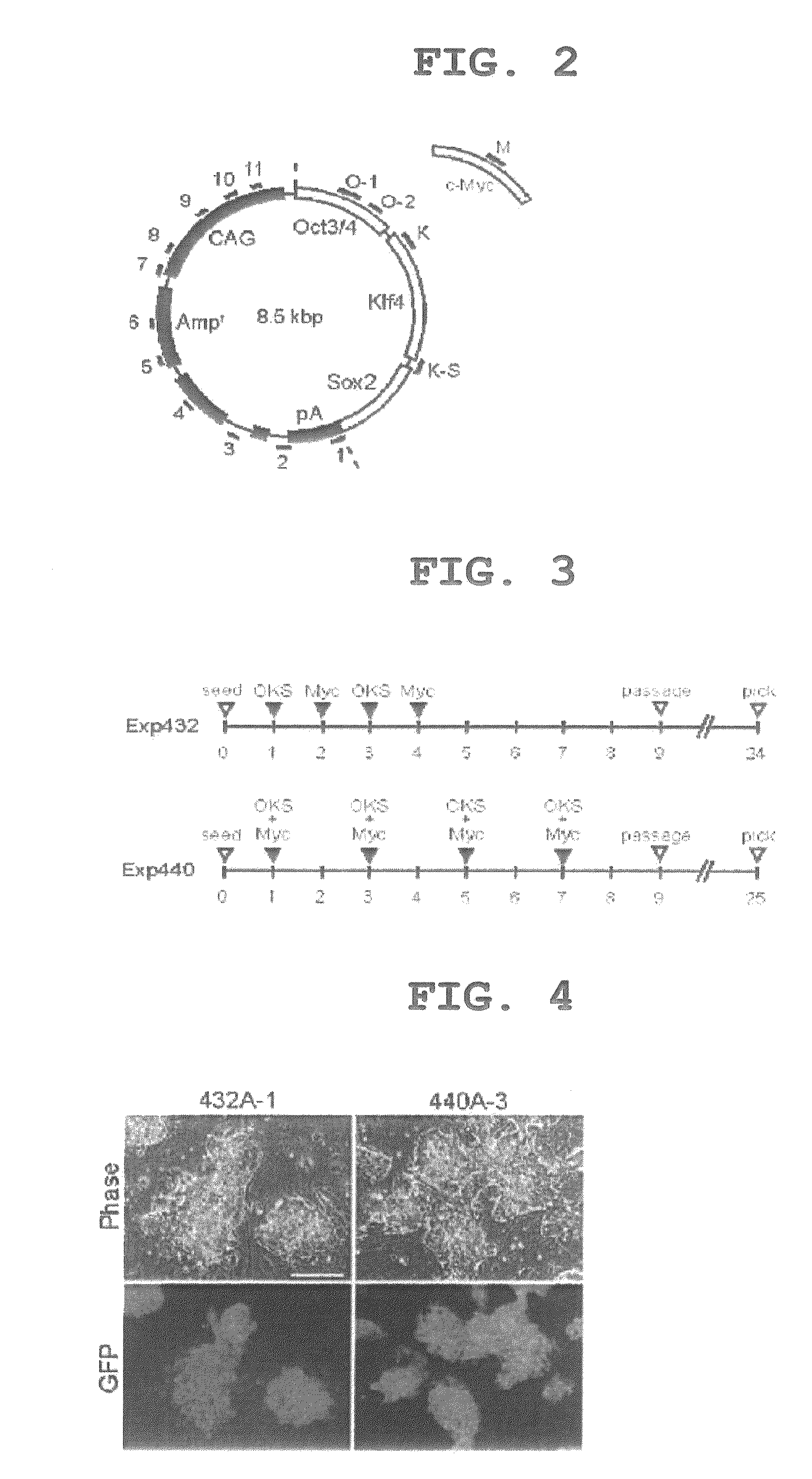
[0145]To avoid the integration of pCX-2A-mOKS and pCX-c-Myc into the host genome, the transfection protocol was modified. On days 1, 3, 5, and 7 after the start of the experiment, pCX-2A-mOKS and pCX-c-Myc were transfected together (Exp440 in FIG. 3). As a result, many GFP-positive colonies were obtained, and cells morphologically indistinguishable from ES cells were produced (440A-3 in FIG. 4). The cells obtained expressed the ES cell markers at the same level as with ES cells (iPS-440A in FIG. 5). To examine for the integration of the plasmid DNA into the genome, 16 sets of PCR primers capable of amplifying each portion of the plasmid were designed (FIGS. 2, 13 and 14). In 9 of the 11 GFP-positive clones obtained by the modified protocol, no amplification of an exogenous DNA was observed (FIG. 6). Furthermore, in Southern blot analysis, no integration of an exogenous gene was detected in these clones (FIG. 11). Although the possible presence of a small plasmid fragment cannot be r...
example 3
[0147]To confirm the pluripotency of iPS cells without integration, iPS cells obtained as described in Example 2 were subcutaneously transplanted to nude mice. All clones tested (440A-3, -4, -8 and -10) produced tumors, which included a broad range of cell types, including cells derived from all the three germ layers (FIG. 7). Furthermore, iPS cells without integration were injected into ICR mouse blastocysts. Judging from the coat colors, adult chimeras were obtained from all clones injected (440A-3, -4, -6, -8, -9 and -10) (FIG. 8). In these chimeric mice, PCR analysis did not detect the integration of any of the transgenes (FIG. 9). The PCR analysis detected both the Nanog and Fbx15 reporters in the chimeras (FIG. 9). Combined with the fact that iPS cells without integration emerged from the double reporter mice, and that the inventor's laboratory does not keep double reporter ES cells, these results showed that the chimeras were derived from iPS cells without integration, rather...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com