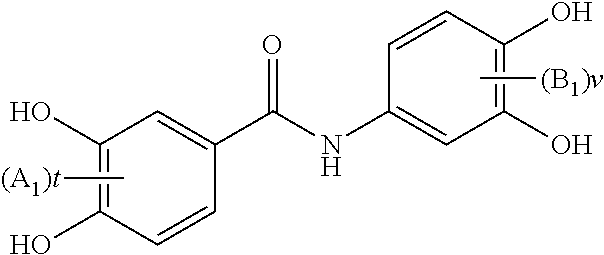
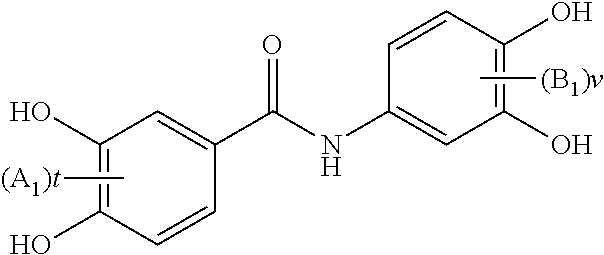
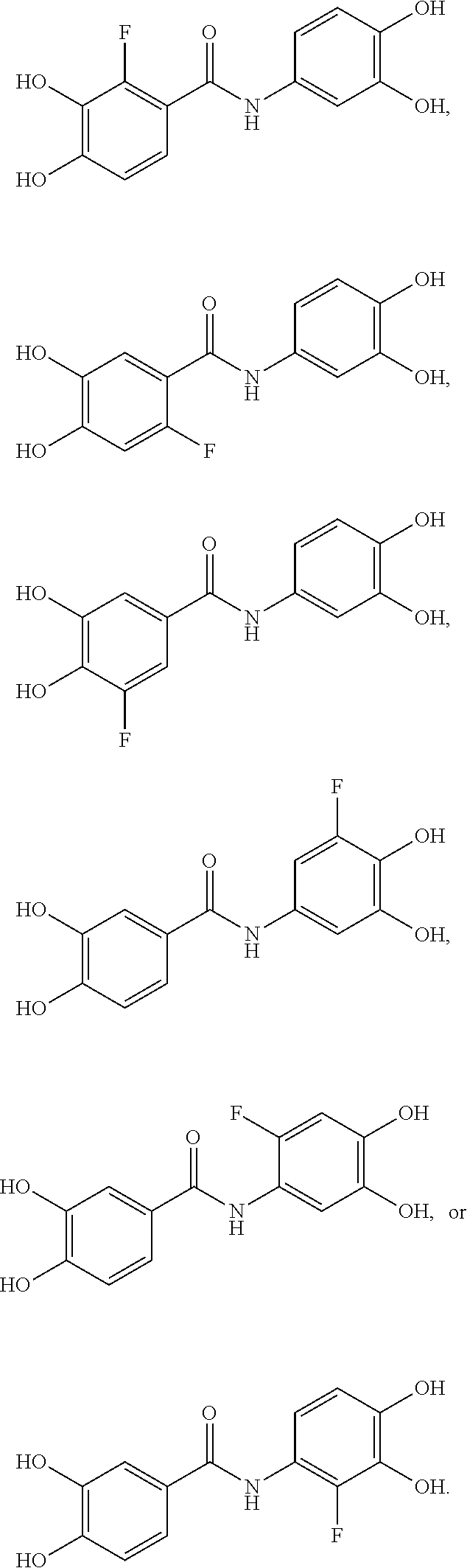
Substituted n-aryl benzamides and related compounds for treatment of amyloid diseases and synucleinopathies
a technology of amyloid disease and synucleinopathies, which is applied in the field of substituted naryl benzamides and related compounds, pharmaceutical compositions, can solve the problems of toxic and neuronal cell death, no cure or effective treatment, and amyloid deposition can be detrimental to the patient, and achieve enhanced efficacy and metabolic optimization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0139]For the following synthetic methods this HPLC method was employed. Samples were analysed using an Agilent HP1100 instrument, operated with EzChrom Elite software, and fitted with a C18 column (Phenomenex Prodigy 5 μm 100A, 250×4.6 mm) with a guard column (Phenomenex ODS 4×3 mm, 5 μm) held at 30° C. Peaks were detected at 280 nm. The mobile phase was acetonitrile in water (with 0.1% TFA): t0=11%, t20=11%, t30=100%, t31=11%, t40=11%. The flow rate was 1 mL / min and the injection volume of 5 μL.
Overview of Synthesis of N-(2-fluoro-4,5-dihydroxyphenyl)-3,4-dihydroxy benzamide (DC51-F5)
[0140]Nitration (I. M. Takakis et al. I Heterocyclic Chem. 1991, 28, 625-634) of commercially available 4-fluoroveratrole gave, as expected, just the 2-nitro isomer 1, as shown by NMR. Reduction using tin II chloride (A. Kamal et al. Bioorg. Med. Chem. 2007, 15 (22), 6868-6875) of this gave the aniline 2, which was immediately reacted with 3,4-methylenedioxybenzoyl chloride to give the anil...
example 2
Overview of Synthesis of Positional isomers N-(3-fluoro-4,5-dihydroxyphenyl)-3,4-dihydroxy benzamide (DC51-F4) and N-(2-fluoro-3,4-dihydroxyphenyl)-3,4-dihydroxy benzamide (DC51-F6)
[0154]3-Fluoroveratrole 4 was prepared by methylation4 of the commercially available 3-fluorocatechol. Nitration of 3-fluoroveratrole gave a mixture of the two isomeric products 5 and 6 in a 2:1 ratio (structures and ratio determined by NMR spectroscopy). Separation of these, followed by reduction with tin II chloride3 gave the anilines 7 and 8. Reaction of the anilines with 3,4-methylenedioxybenzoyl chloride gave the anilides 9 and 10. Deprotection with boron tribromide under standard conditions gave the free phenolic anilides, N-(3-fluoro-4,5-dihydroxyphenyl)-3,4-dihydroxybenzamide (DC51-F4) and N-(2-fluoro-3,4-dihydroxyphenyl)-3,4-dihydroxybenzamide (DC51-F6) in reasonable yield.
Step A: Preparation of 1-fluoro-2,3-dimethoxy-5-nitrobenzene (5) and 1-fluoro-2,3-dimethoxy-6-nitrobenzene (6)
[0155]To ice co...
example 3
Overview of Synthesis of N-(3,4-dihydroxyphenyl)-2-fluoro-4,5-dihydroxy benzamide (DC51-F2)
[0178]Oxidation of commercially available 6-fluoroveratraldehyde with ‘Jones reagent'6 gave the acid 11 in good yield. Formation of’ the acid chloride from this and condensation with 3,4-methylenedioxyaniline then gave the anilide 12. Deprotection with boron tribromide under standard conditions gave the free phenolic anilide, DC51-F2 in good yield.
Step A: Preparation of 2-fluoro-4,5-dimethoxybenzoic acid (11)
[0179]To a stirred solution of 2-fluoro-4,5-dimethoxybenzaldehyde (0.46 g, 2.5 mmol) in acetone (20 ml) was added Jones reagent (6 ml) dropwise and the mixture stirred at RT for 4 h (P. B. Wakchaure et al. Tetrahedron 2008, 64, 1786-1791.). The mixture was diluted with water, extracted into ethyl acetate then dried and evaporated in vacuo to give the crude acid. Recrystallisation from ethyl acetate / pet ether (40-60) gave the pure acid as a brown crystalline solid (H. B. Stegmann et al. J.C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| body weight | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com